chair conformation explained Best chair configuration for trimethylcyclohexane matching a specific Haworth projection. In marking thousands of exam papers theres 2 mistakes that Ive seen over and over again.
Chair Conformation Explained, In fact the appropriate word conforma-tion had already been used in sugar chemistry by W. In the chair conformation there are two planes with carbon atoms alternating making carbon atoms 1 3 and 5 in one plane and carbon atoms 2 4 and 6 in another plane. Alternate your axial substituents up and down all the way around your cyclohexane.
 Chair Conformation And Ring Flips Youtube From youtube.com
Chair Conformation And Ring Flips Youtube From youtube.com
To begin start by drawing two lines that are parallel to each other but not perfectly horizontal as shown here. And now the stabilities. The most general definition of conformation is as follows 7. Rather than use the vague terms boat and chair forms of cyclohexane it is convenient to have a general term. This is a multistep process so here Im going to walk you through it from scratch.
The price to you remains the same.
Every carbon on the chair conformation has. Ho OH OH explaining why the conformation witlh The-OH in the axial position is the lower energy conformation. Next add a downward-pointing V tip to one end this is the tail of the chair. Chair conformations are commonly used to describe the various interactions between atoms on cycylohexanes. To begin start by drawing two lines that are parallel to each other but not perfectly horizontal as shown here.
Another Article :
 Source: chemistrysteps.com
Source: chemistrysteps.com
Anti-periplanar Elimination Explained. In the boat conformation two of the substituents those on the bow and the stern if you will are brought close enough to each other to cause steric strain. But to draw them naturally to get them flowing off your pen and onto the page perfectly at exam time youre gonna need to practice. In each of the conformations drawn in step 4 circle the axial substituents other than hydrogen. We will explore anti-periplanar elimination. And now the stabilities. Ring Flip Of Chair Conformations With Practice Problems Chemistry Steps.
 Source: youtube.com
Source: youtube.com
For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. This means that the chair conformation is the structure that is observable in most reactions of cyclohexane. In the chair conformation there are two planes with carbon atoms alternating making carbon atoms 1 3 and 5 in one plane and carbon atoms 2 4 and 6 in another plane. In the boat conformation two of the substituents those on the bow and the stern if you will are brought close enough to each other to cause steric strain. This is a multistep process so here Im going to walk you through it from scratch. To begin start by drawing two lines that are parallel to each other but not perfectly horizontal as shown here. Anti Periplanar Elimination Explained Chair Conformation Mechanism Mcat Organic Chemistry Youtube.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
This is because it has low energy. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to. We will explore anti-periplanar elimination. Chair conformations are commonly used to describe the various interactions between atoms on cycylohexanes. Rather than use the vague terms boat and chair forms of cyclohexane it is convenient to have a general term. For each chair conformer add the energy of all the groups on axial position. 3 6 Conformations Of Cyclic Alkanes Organic Chemistry 1 An Open Textbook.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
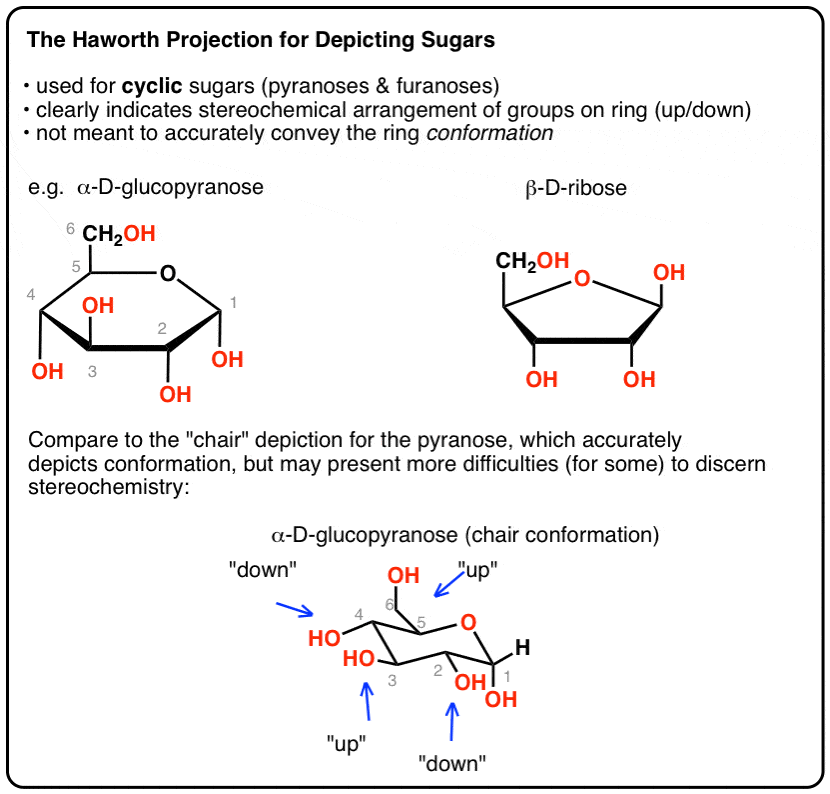
This means that the chair conformation is the structure that is observable in most reactions of cyclohexane. We will explore anti-periplanar elimination. If the substituent is in axial position when exhibiting a 13 interaction then the chair conformation is in higher energy due to high electron. The chair conformation is free of torsional strain as well. In each of the conformations drawn in step 4 circle the axial substituents other than hydrogen. And now the stabilities. The Haworth Projection Master Organic Chemistry.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
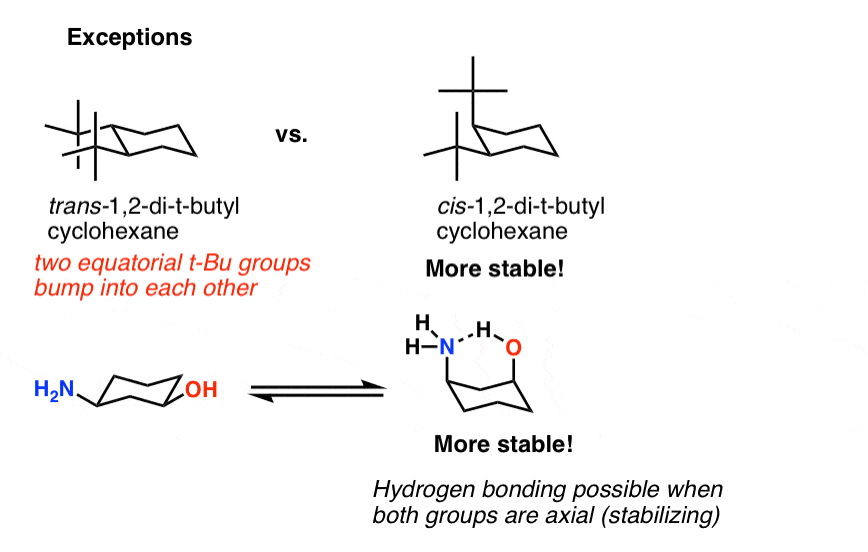
In fact the appropriate word conforma-tion had already been used in sugar chemistry by W. However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solution. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. The chair conformation is considered to be the lowest energy conformation because it lacks both angle strain and torsional strain. If the substituent is in axial position when exhibiting a 13 interaction then the chair conformation is in higher energy due to high electron. Draw the second chair conformation ring-flip-check this post if not sure. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
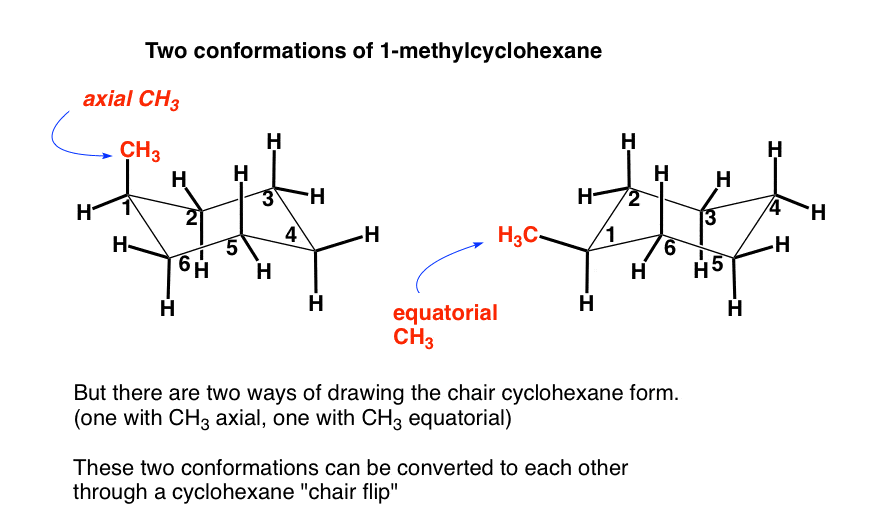
Chair conformation is the most stable structure of cyclohexane. In marking thousands of exam papers theres 2 mistakes that Ive seen over and over again. What is Chair Conformation. This is a multistep process so here Im going to walk you through it from scratch. Once youve mastered the art of drawing chair conformations its time to stick some axial and equatorial substituents on those beautiful chairs. Ho OH OH explaining why the conformation witlh The-OH in the axial position is the lower energy conformation. The Cyclohexane Chair Flip Master Organic Chemistry.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
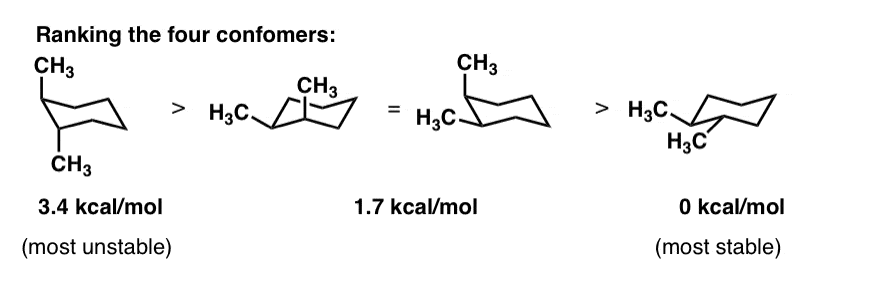
Anti-periplanar Elimination Explained. In the chair conformation there are two planes with carbon atoms alternating making carbon atoms 1 3 and 5 in one plane and carbon atoms 2 4 and 6 in another plane. Every carbon on the chair conformation has. If the substituent is in axial position when exhibiting a 13 interaction then the chair conformation is in higher energy due to high electron. The steps involved in drawing the chair conformation of cyclohexane. Chair conformations are commonly used to describe the various interactions between atoms on cycylohexanes. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Chair Conformation Mechanism MCAT Organic Chemistry - YouTube. We will explore anti-periplanar elimination. However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solution. Next add a downward-pointing V tip to one end this is the tail of the chair. The price to you remains the same. What is Chair Conformation. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
 Source: youtube.com
Source: youtube.com
In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. This means that the chair conformation is the structure that is observable in most reactions of cyclohexane. However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solution. The most general definition of conformation is as follows 7. Chair Conformation Mechanism MCAT Organic Chemistry - YouTube. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to. Chair Conformation And Ring Flips Youtube.
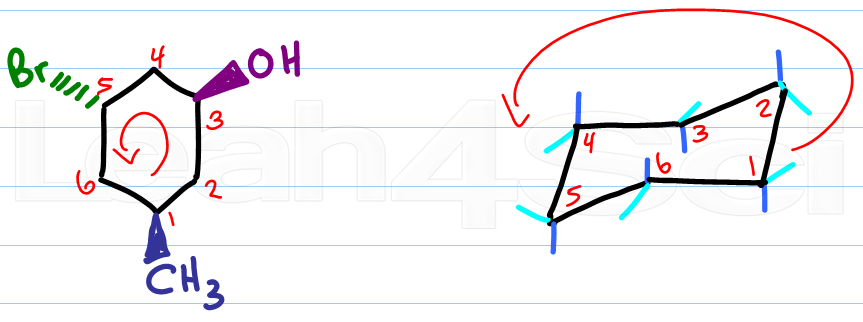 Source: leah4sci.com
Source: leah4sci.com
In the boat conformation two of the substituents those on the bow and the stern if you will are brought close enough to each other to cause steric strain. Just three pairs of parallel lines and youve drawn a perfect chair conformation. This is true for 1R-33-dichlorocyclohexanol. In the boat conformation two of the substituents those on the bow and the stern if you will are brought close enough to each other to cause steric strain. Chair Conformation Mechanism MCAT Organic Chemistry - YouTube. To begin start by drawing two lines that are parallel to each other but not perfectly horizontal as shown here. Drawing Chair Conformations And Ring Flips For Cyclohexane In Organic Chemistry.
 Source: youtube.com
Source: youtube.com
An alternate conformation for a six-membered ring is called the boat. Anti-periplanar Elimination Explained. Ho OH OH explaining why the conformation witlh The-OH in the axial position is the lower energy conformation. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. The steps involved in drawing the chair conformation of cyclohexane. Consider the interactions that typically make the axial position more crowded and higher energy. Chair Conformations Examples Youtube.
 Source: leah4sci.com
Source: leah4sci.com
The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to. This is because it has low energy. The steps involved in drawing the chair conformation of cyclohexane. And now the stabilities. Number the ring and draw any chair conformation of the compound. To begin start by drawing two lines that are parallel to each other but not perfectly horizontal as shown here. Drawing Chair Conformations And Ring Flips For Cyclohexane In Organic Chemistry.
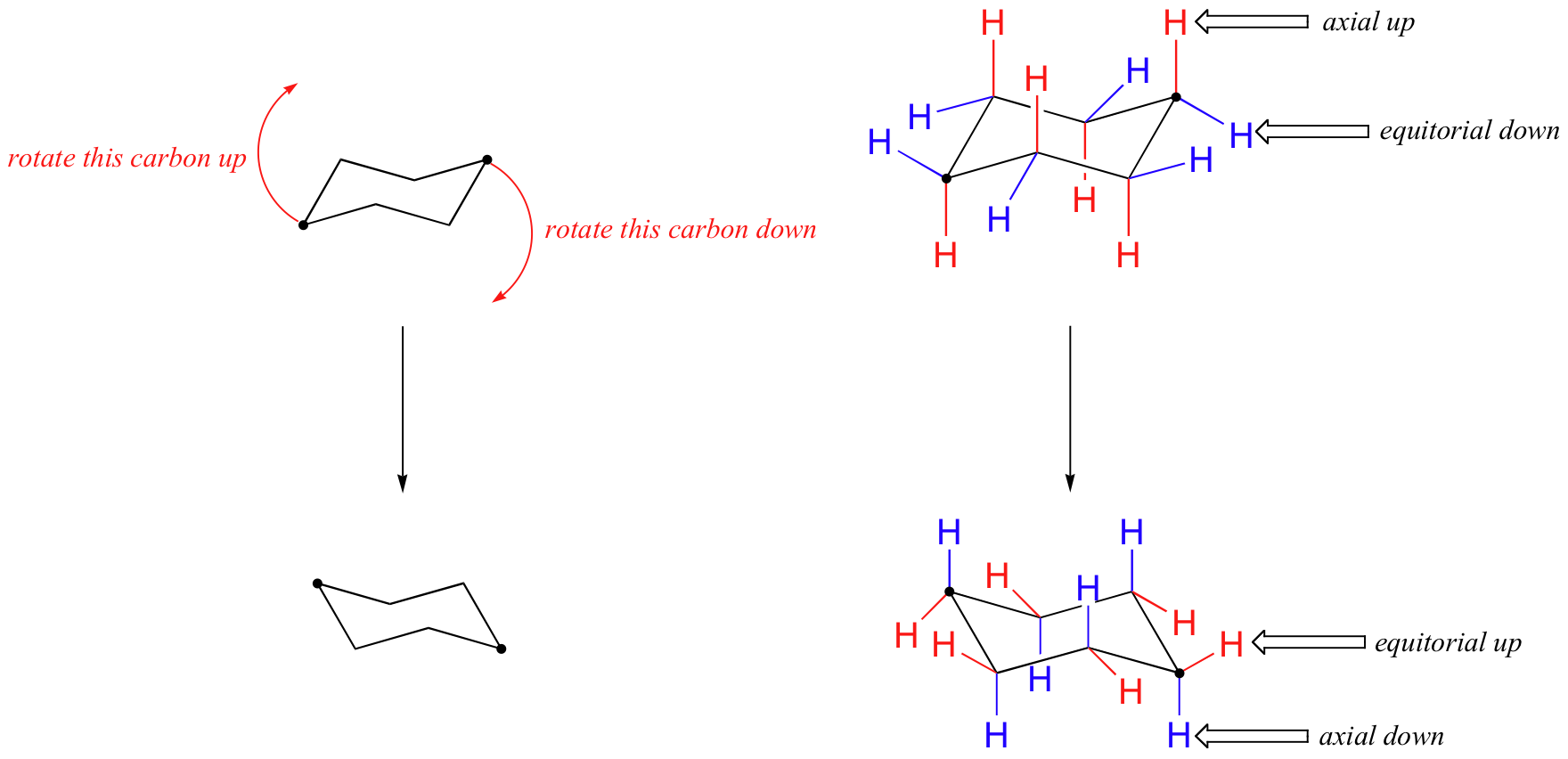 Source: chem.libretexts.org
Source: chem.libretexts.org
Chair conformation is the most stable structure of cyclohexane. In each of the conformations drawn in step 4 circle the axial substituents other than hydrogen. Finally add an upward-pointing V tip to the other end this is the nose of the chair. In fact the appropriate word conforma-tion had already been used in sugar chemistry by W. The 13 describes the distance between the substituent and hydrogens that are in axial position. This is because it has low energy. 4 7 Cyclohexane Conformations Chemistry Libretexts.
 Source: youtube.com
Source: youtube.com
In the chair conformation there are two planes with carbon atoms alternating making carbon atoms 1 3 and 5 in one plane and carbon atoms 2 4 and 6 in another plane. Anti-periplanar Elimination Explained. For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. In the chair conformation there are two planes with carbon atoms alternating making carbon atoms 1 3 and 5 in one plane and carbon atoms 2 4 and 6 in another plane. In fact the appropriate word conforma-tion had already been used in sugar chemistry by W. But to draw them naturally to get them flowing off your pen and onto the page perfectly at exam time youre gonna need to practice. How To Draw Cyclohexane Chair Conformations And Ring Flips Youtube.
4 Draw the two chair conformations of the heterocycle shown on the left. The price to you remains the same. Chair conformations are commonly used to describe the various interactions between atoms on cycylohexanes. For each chair conformer add the energy of all the groups on axial position. Number the ring and draw any chair conformation of the compound. Heres how to avoid those mistakes ace any chair conformations in your OChem. Chair And Boat Shapes For Cyclohexane Video Khan Academy.










