unstable chair conformation The chair conformation is the most stable conformation of cyclohexane. Why is chair conformation of cyclohexane more stable than boat form.
Unstable Chair Conformation, Thus 3 and 5 are both equally stable and they are the most stable conformations for 2-methylbutane. In both conformations chair and boat the bond angle is 1095 which eliminates the angular strain in the cyclohexane structure. The answer is because of the torsional and steric strains which are more present in a boat conformation.
 Dzhd Liniya Na Sajta Drebna Riba Most Stable Chair Conformation Tricomfireprotection Com From tricomfireprotection.com
Dzhd Liniya Na Sajta Drebna Riba Most Stable Chair Conformation Tricomfireprotection Com From tricomfireprotection.com
So the equatorial conformation is more stable than the axial by 728 kJmol. For this class we will always find that the most stable conformation is staggered ie 1 3 or 5 and the least stable is eclipsed ie 2 4 or 6. So the stability increases from Half chair to boat to twist boat and finally the chair conformation. And now the stabilities. A second much less stable conformer is the boat conformation.
So the stability increases from Half chair to boat to twist boat and finally the chair conformation.
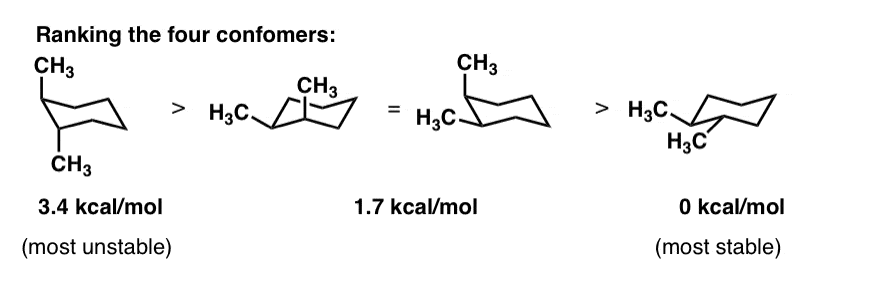
As you can see as the groups get bigger ethyl tertbutyl these values start to get really crazy high. As you can see as the groups get bigger ethyl tertbutyl these values start to get really crazy high. The minimum energy conformation of your example is shown below. The energy barriers between the chair boat and twist conformations of cyclohexane are low enough Fig6 to make separation of the. Of these two positions of the Hs the equitorial form will be the most stable because the hydrogen atoms or perhaps the.
Another Article :
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
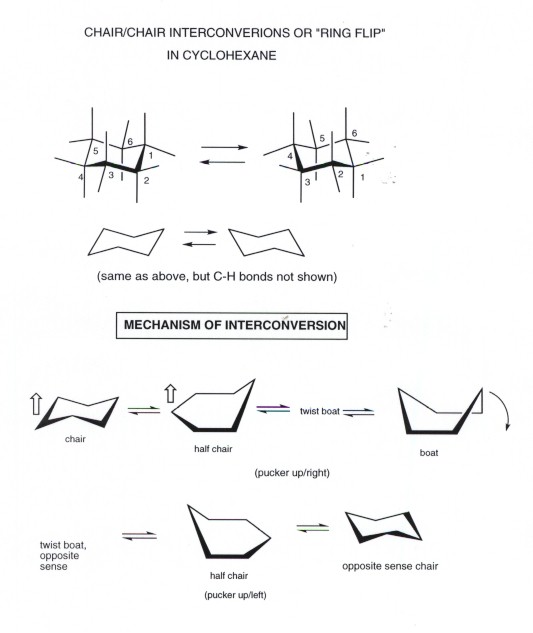
Firstly Newman projections along either of the horizontal C-C bonds in the boat form show eclipsing and secondly the two hydrogens shown the socalled bowsprit hydrogens come within such a short distance of one another that a repulsive force. The chair conformation is the most stable conformation of cyclohexane. The different conformations are called conformers a blend of the words conformation and isomer. The chair conformation is more stable because it does not have any steric hindrance or steric repulsion between the hydrogen bonds. And using the ratio 955 it is calculated that this corresponds to 728 kJmol. This energy difference is known as the A value and it varies depending on the axial group. Most Stable Chair Conformation Chemistry Stack Exchange.
 Source: research.cm.utexas.edu
Source: research.cm.utexas.edu
A chair conformation is one of many conformations of a cyclohexane ring and it is most stable. I n chair cyclohexane there are two types of positions axial and equatorial. It has equatorial and axial bonds. The larger the group the higher the energy difference. Cyclohexane has two limiting conformations the chair and the boat. The chair conformation is the most stable conformer. Cyclohexane Conformational Analysis.
 Source: research.cm.utexas.edu
Source: research.cm.utexas.edu
The equatorial bonds e are perpendicular to the axis of the ring while axial bonds a are parallel to the axis of the ring. Cyclohexane and the natural bond angles of C C and C H add a great deal of flexibility to the ring. Stability of conformation decided by many factors like angle strain- any deviation from normal bond angles. The larger the group the higher the energy difference. Besides boat conformation tends to convert into the boat-twist conformation. Number the ring and draw any chair conformation of the compound. Cyclohexane Conformational Analysis.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
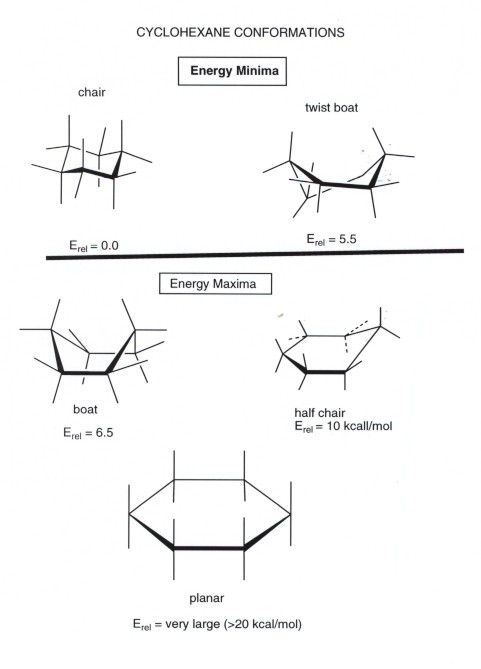
Torsional strain- a pair of tetrahedral carbons attached to each other have their bond staggered. The process of ring flipping takes cyclohexane through a conformation called the twist chair or the half chair form and this form is 108kcal less stable than the chair conformer. The chair conformation is estimated to be lower in energy than the twist conformation by approximately 23 kJ mol-1. Chair conformation of cyclohexane each carbon atom has one axial and one equatorial hydrogen atom correctly describes the the bond angles are near 1095 correctly describes the. Cyclohexane has two limiting conformations the chair and the boat. The minimum energy conformation of your example is shown below. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
 Source: tricomfireprotection.com
Source: tricomfireprotection.com
The chair conformation is the most stable conformer. And using the ratio 955 it is calculated that this corresponds to 728 kJmol. The chair conformation is more stable because it does not have any steric hindrance or steric repulsion between the hydrogen bonds. The minimum energy conformation of your example is shown below. Of these two positions of the Hs the equitorial form will be the most stable because the hydrogen atoms or perhaps the. The axial positions point perpendicular to the plane of the ring whereas the equatorial positions are around the plane of the ring. Dzhd Liniya Na Sajta Drebna Riba Most Stable Chair Conformation Tricomfireprotection Com.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Expand the ring to 6 members ie. Usually chair conformation is the most stable conformation and at room temperature about 9999 of cyclohexane in a mixture of different conformation exists in this conformation. The equatorial bonds e are perpendicular to the axis of the ring while axial bonds a are parallel to the axis of the ring. A second much less stable conformer is the boat conformation. Comparing 1 3 and 5 we see that 1 has two bad gauche interactions whereas 3 and 5 have only one gauche interaction. It has equatorial and axial bonds. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
 Source: byjus.com
Source: byjus.com
Chair conformation of cyclohexane each carbon atom has one axial and one equatorial hydrogen atom correctly describes the the bond angles are near 1095 correctly describes the. The equatorial bonds e are perpendicular to the axis of the ring while axial bonds a are parallel to the axis of the ring. It has equatorial and axial bonds. Comparing 1 3 and 5 we see that 1 has two bad gauche interactions whereas 3 and 5 have only one gauche interaction. This too is almost free of angle strain but in contrast has torsional strain associated with eclipsed bonds at the four of the C atoms that form the side of the boat. Answer 1 of 3. Conformation Of Cyclohexane Chair Twist Boat Boat And Half Chair.
 Source: tricomfireprotection.com
Source: tricomfireprotection.com
The chair conformation is the most stable conformation of cyclohexane. Firstly Newman projections along either of the horizontal C-C bonds in the boat form show eclipsing and secondly the two hydrogens shown the socalled bowsprit hydrogens come within such a short distance of one another that a repulsive force. Thus 3 and 5 are both equally stable and they are the most stable conformations for 2-methylbutane. Stability of conformation decided by many factors like angle strain- any deviation from normal bond angles. The chair conformation is the most stable conformation of cyclohexane. Answer 1 of 3. Dzhd Liniya Na Sajta Drebna Riba Most Stable Chair Conformation Tricomfireprotection Com.
 Source: chemistrysteps.com
Source: chemistrysteps.com
As you can see as the groups get bigger ethyl tertbutyl these values start to get really crazy high. It has equatorial and axial bonds. I n chair cyclohexane there are two types of positions axial and equatorial. Due to this reason chair conformation is stable than boat conformation. This energy difference is known as the A value and it varies depending on the axial group. However why chair is more stable than boat. Ring Flip Comparing The Stability Of Chair Conformations With Practice Problems Chemistry Steps.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
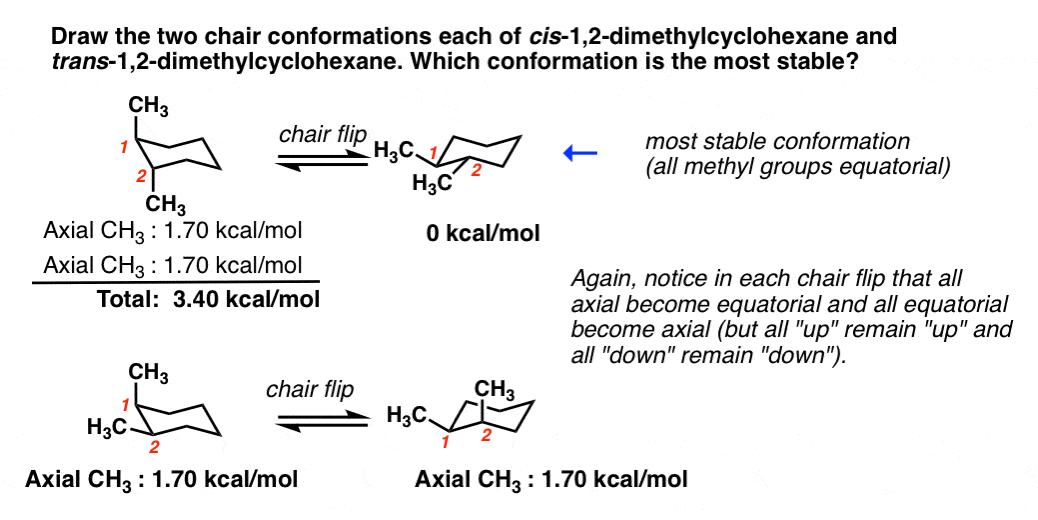
Usually chair conformation is the most stable conformation and at room temperature about 9999 of cyclohexane in a mixture of different conformation exists in this conformation. Expand the ring to 6 members ie. Draw the second chair conformation ring-flip-check this post if not sure. Cyclohexane has two limiting conformations the chair and the boat. The chair conformation is estimated to be lower in energy than the twist conformation by approximately 23 kJ mol-1. It has equatorial and axial bonds. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
 Source: youtube.com
Source: youtube.com
I ran a few simple calculations molecular mechanics followed by DFT optimisation - gas phase on the axial and equatorial conformations which showed that the two conformations are separated by around 34 kcalmol. Besides boat conformation tends to convert into the boat-twist conformation. And now the stabilities. This too is almost free of angle strain but in contrast has torsional strain associated with eclipsed bonds at the four of the C atoms that form the side of the boat. The minimum energy conformation of your example is shown below. Chair conformation of cyclohexane is more stable than boat form because in chair conformaion the C-H bonds are equally axial and equatorial ie out of twelve C-H bonds six are axial and six are equatorial and each carbon has one axial and one equatorial C-H bond. Evaluating Relative Stability Of Chair Conformers Youtube.
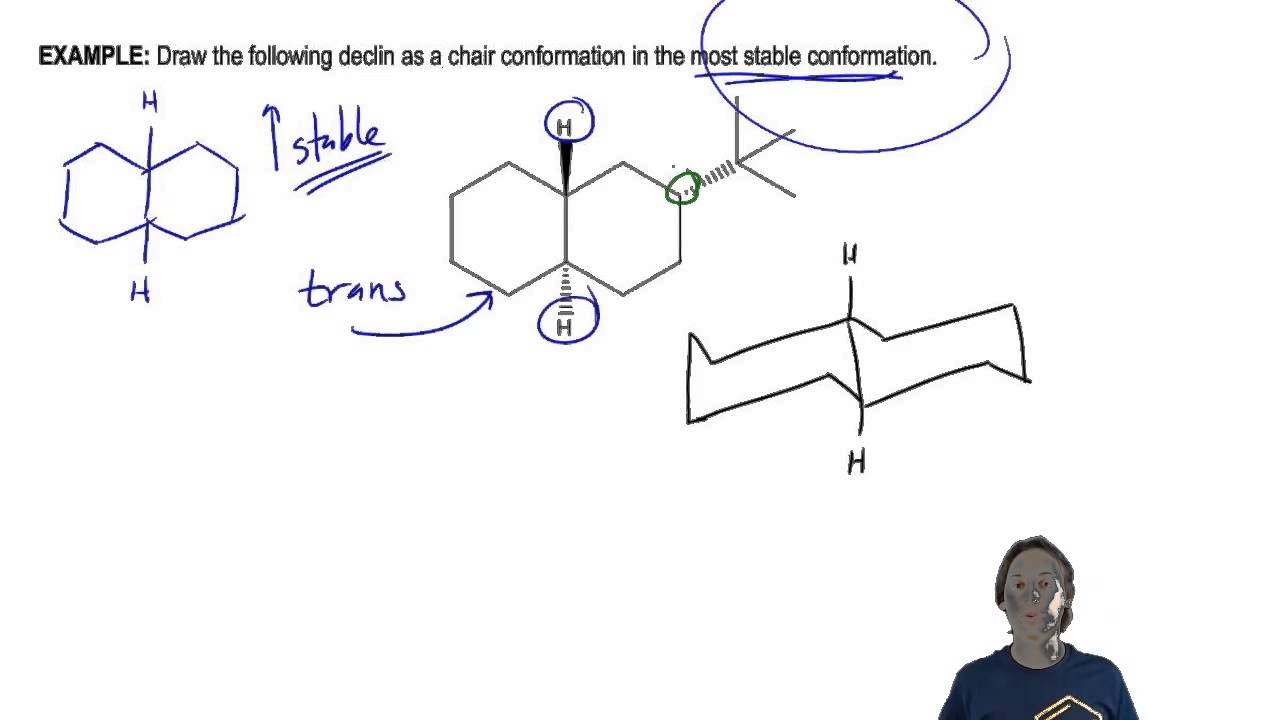 Source: tricomfireprotection.com
Source: tricomfireprotection.com
At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. Draw the second chair conformation ring-flip-check this post if not sure. Answer 1 of 3. Besides boat conformation tends to convert into the boat-twist conformation. A chair conformation is one of many conformations of a cyclohexane ring and it is most stable. However why chair is more stable than boat. Dzhd Liniya Na Sajta Drebna Riba Most Stable Chair Conformation Tricomfireprotection Com.
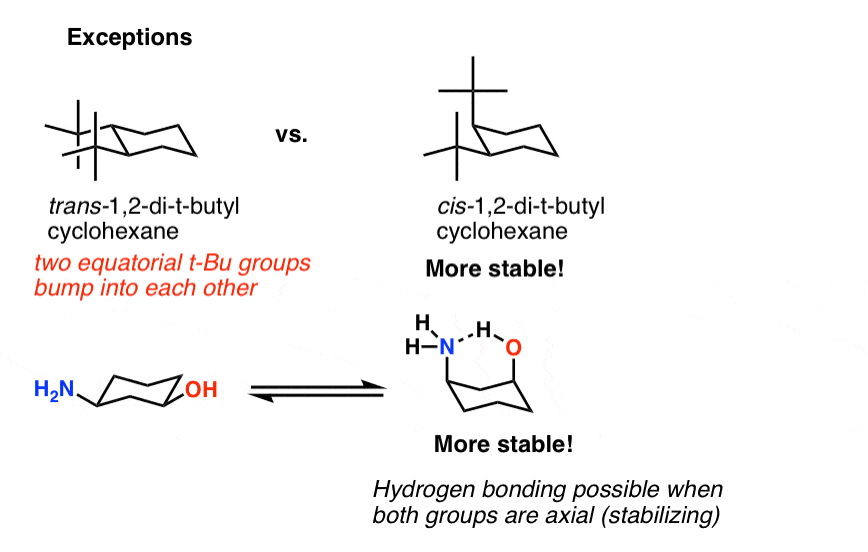 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Comparing 1 3 and 5 we see that 1 has two bad gauche interactions whereas 3 and 5 have only one gauche interaction. So the equatorial conformation is more stable than the axial by 728 kJmol. Chair conformation of cyclohexane is more stable than boat form because in chair conformaion the C-H bonds are equally axial and equatorial ie out of twelve C-H bonds six are axial and six are equatorial and each carbon has one axial and one equatorial C-H bond. A chair conformation is one of many conformations of a cyclohexane ring and it is most stable. The chair conformation that you have drawn 4 C 1 is likely to be the most stable one as it minimizes the number of heavy axial groupsTo determine the chair conformation of a hexose it is generally easiest to draw it and compare it with β-D-glucose where all heavy groups are equatorial and the conformation is 4 C 1If the number of heavy axial groups becomes smaller when the conformation. So the stability increases from Half chair to boat to twist boat and finally the chair conformation. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
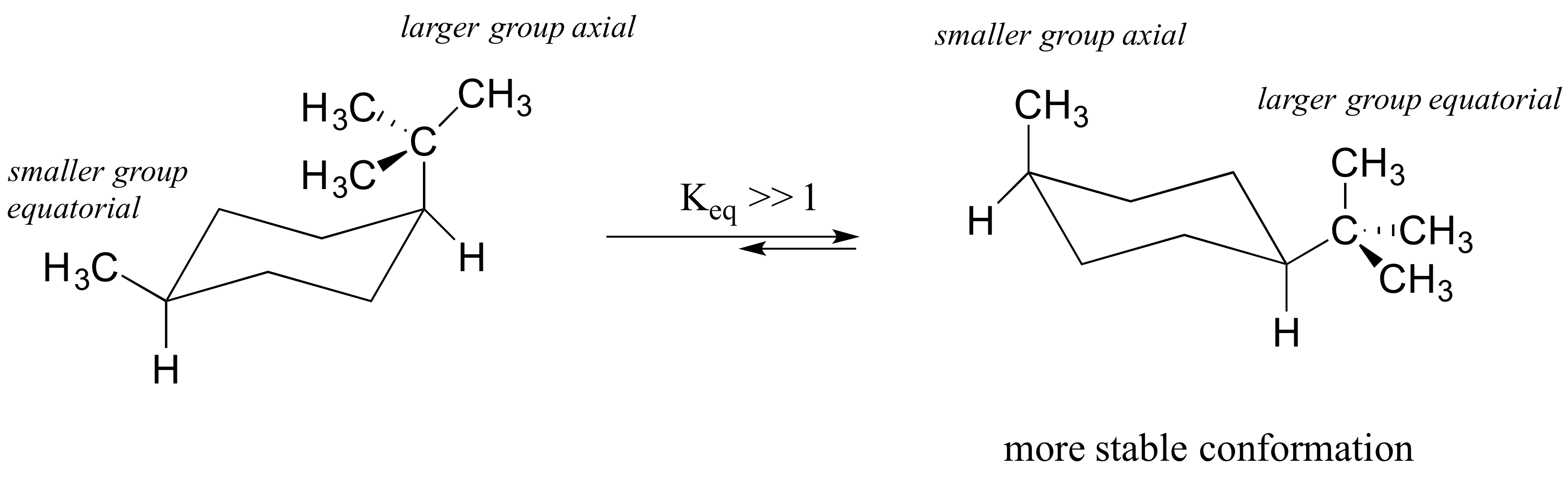 Source: tricomfireprotection.com
Source: tricomfireprotection.com
For example the energy difference of the axial ethyl cyclohexane with the equatorial conformer is 73 kJmol. Firstly Newman projections along either of the horizontal C-C bonds in the boat form show eclipsing and secondly the two hydrogens shown the socalled bowsprit hydrogens come within such a short distance of one another that a repulsive force. Answer 1 of 3. The different conformations are called conformers a blend of the words conformation and isomer. For example the energy difference of the axial ethyl cyclohexane with the equatorial conformer is 73 kJmol. At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. Dzhd Liniya Na Sajta Drebna Riba Most Stable Chair Conformation Tricomfireprotection Com.
 Source: tricomfireprotection.com
Source: tricomfireprotection.com
This too is almost free of angle strain but in contrast has torsional strain associated with eclipsed bonds at the four of the C atoms that form the side of the boat. A second much less stable conformer is the boat conformation. Why is chair conformation of cyclohexane more stable than boat form. Chair conformation of cyclohexane each carbon atom has one axial and one equatorial hydrogen atom correctly describes the the bond angles are near 1095 correctly describes the. The minimum energy conformation of your example is shown below. Just so you guys know 23 kilojoules per mole is a large number in. Dzhd Liniya Na Sajta Drebna Riba Most Stable Chair Conformation Tricomfireprotection Com.










