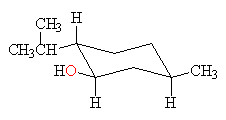
neomenthol chair conformation Organic Chemistry 2nd Edition Edit edition Solutions for Chapter 6 Problem 26AP. It was shown by NMR spectroscopy gas phase electron diffraction and several quantum chemical computation studies that the chair conformation of cyclohexane is preferred in menthol Egawa et al 2003.
Neomenthol Chair Conformation, This conformation of cyclohexane is called the chair conformation be-cause of its resemblance to a lawn chair. The chair confirmation off methanol is soon as follows. Draw the following chair in the most stable conformation.
 Menthol And Neomenthol From askthenerd.com
Menthol And Neomenthol From askthenerd.com
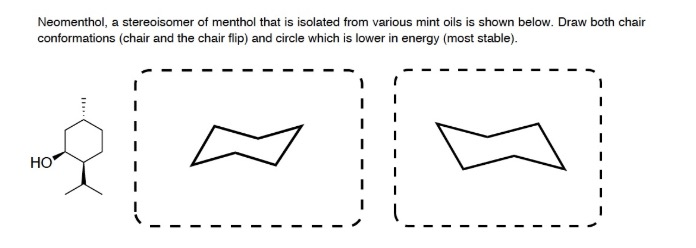
Here it is can see this structure. For each chair conformer add the energy of all the groups on axial position. Neomenthol a stereoisomer of menthol that is isolated from various mint oils is shown below. On this is ring flip. In these structures the hydroxyl group takes an axial orientation.
You can see now the first year confirmation has less 13 die actual interaction between the age group as you can see in the structure and.
Number the ring and draw any chair conformation of the compound. B Neomenthol is a stereoisomer of menthol. For neoisomenthol MD simulations showed two chair conformations. There are different ways of drawing a chair conformation and you are free to choose the one you like as long as at the end you have the structures correct. Substituents represented on wedges are always positioned.
Another Article :
 Source: idop2science8.pbworks.com
Source: idop2science8.pbworks.com
If you have not already done so you should. Conformational Analysis which is currently in the final stages of development. Identify the up tip OR down tip of your chair conformation and draw a straight line up up tip or down down tip parallel to the y-plane. The boat forms You can also draw two flipped boat conformations. This conformation of cyclohexane is called the chair conformation be-cause of its resemblance to a lawn chair. However in contrast to calculated energies and correlations between theoretical and experimental 13 C chemical shifts the measured 3 J H3H2 coupling of 63 Hz indicates an equally populated equilibrium of both conformers. Idop2science8 Licensed For Non Commercial Use Only Menthol And The Nervous System.
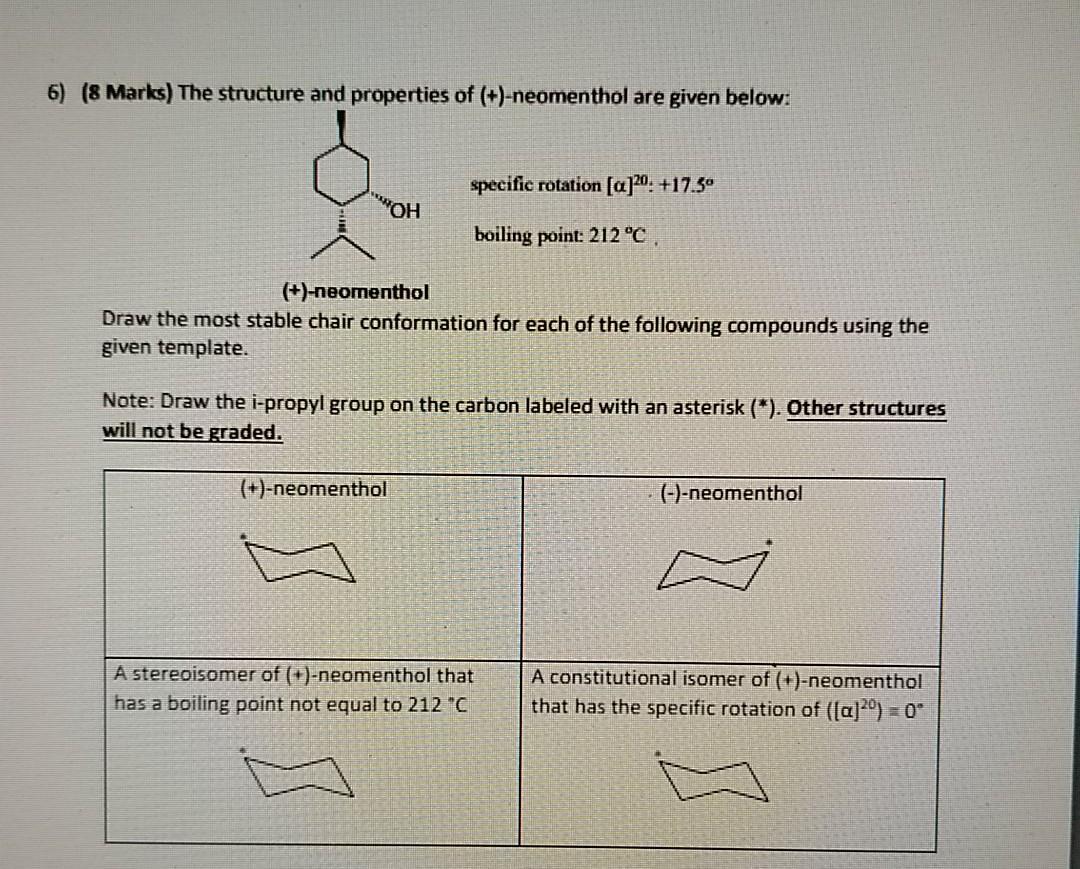
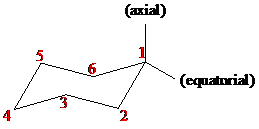
The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to. The chair confirmation off methanol is soon as follows. Identify the up tip OR down tip of your chair conformation and draw a straight line up up tip or down down tip parallel to the y-plane. Scheme in the chair conformation templates drawn in step 3 fill in the substituents on the chair conformations. - - - - - - - - - - - - - - - - - - - - Question. The main form of menthol occurring in nature is -menthol which is assigned the configuration. Solved 6 8 Marks The Structure And Properties Of Chegg Com.
 Source: brainly.com
Source: brainly.com
You can see now the first year confirmation has less 13 die actual interaction between the age group as you can see in the structure and. Draw both chair conformations chair and the chair flip and circle which is lower in energy most stable. That is the specific structure for cyclohexane. It was shown by NMR spectroscopy gas phase electron diffraction and several quantum chemical computation studies that the chair conformation of cyclohexane is preferred in menthol Egawa et al 2003. Neomenthol a stereoisomer of menthol. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to. Menthol Used To Flavor Various Foods And Tobacco Is The Most Stable Stereoisomer Of Brainly Com.
 Source: ursula.chem.yale.edu
Source: ursula.chem.yale.edu
Like in given figure no. Now lets go into more details. Use the guidelines below place substituents in the proper axialequatorial orientation. On this is ring flip. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to. Now draw both of the chair conformations of this molecule and circle the more stable conformation equatiorial preference energies. Final 01.
 Source: chegg.com
Source: chegg.com
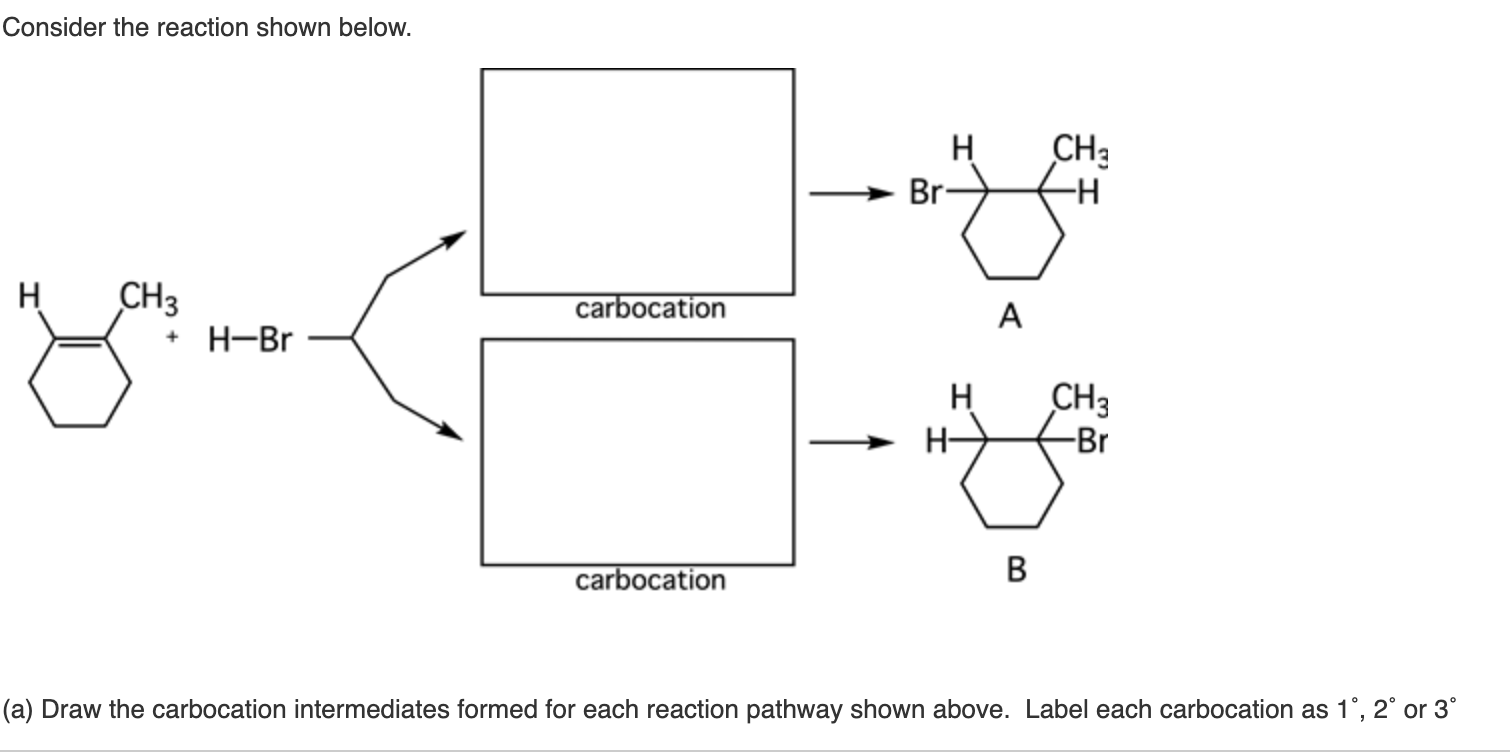
A sample of our newest video. Scheme in the chair conformation templates drawn in step 3 fill in the substituents on the chair conformations. It is a waxy crystalline substance clear or white in color which is solid at room temperature and melts slightly above. And now the stabilities. In the first conformer we have two chlorines in axial positions so the total steric strain is. Now draw both of the chair conformations of this molecule and circle the more stable conformation equatiorial preference energies. Solved The Structure Of Neomenthol Is Drawn Below On A Chegg Com.
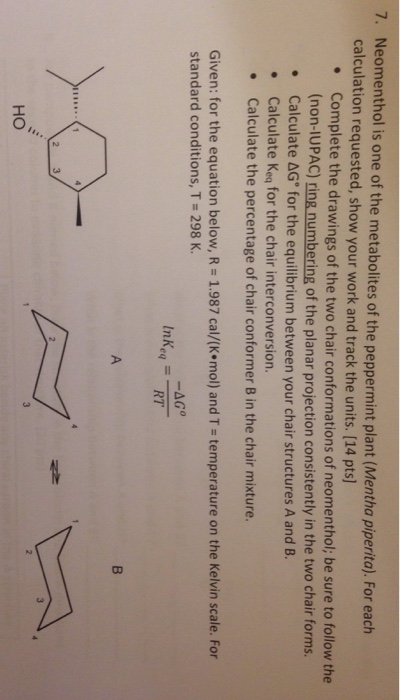
On this is ring flip. Draw the second chair conformation ring-flip-check this post if not sure. That is the specific structure for cyclohexane. This is your first axial substituent. L in this conformation each has 2 axial and 2 equotrial the menthol stereo isomer is more stable by 36 kcalmol. In order to get an overview of the potential energy surface we performed a relaxed two dimensional scan at the B3LYP3-21G level of theory by rotating the isopropyl. Neomenthol Is One Of The Metabolites Of The Chegg Com.
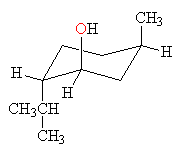 Source: askthenerd.com
Source: askthenerd.com
Härtner and Reinscheid 2008. A potential energy diagram for nng inversion m cyclohexane is shown m Figure 3 18 In the first step the chair conformation is converted to a skew boat which then proceeds to the inverted chair m the second step The skew boat conformation is an inter mediate in the process of ring inversion Unlike a transition state an intermediate is not a. Now draw both of the chair conformations of this molecule and circle the more stable conformation equatiorial preference energies. 1 if both methyl groups. Like in given figure no. It is one of several new videos exploring the c. Menthol And Neomenthol.
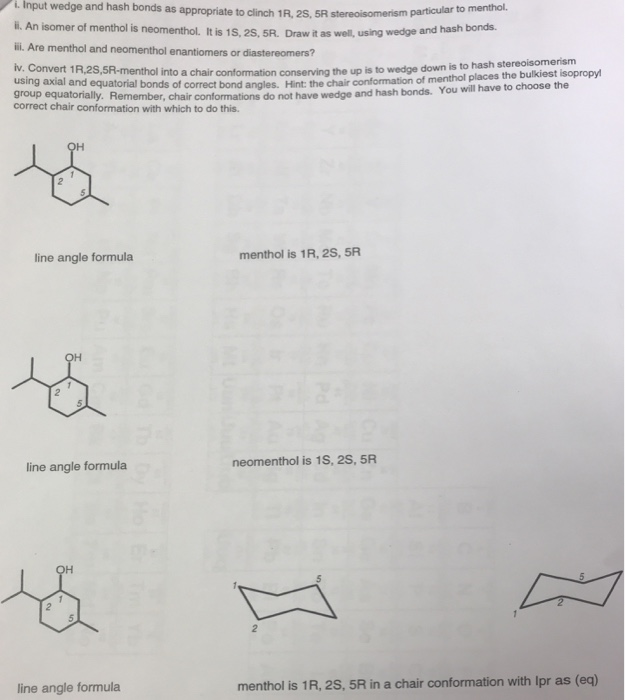 Source: chegg.com
Source: chegg.com
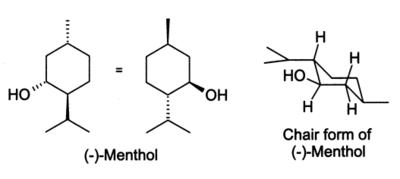
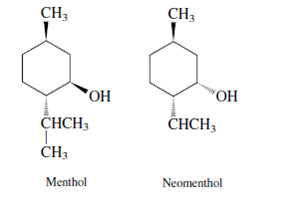
The chair reference is just referring to the repeating zig-zag shape across the longitudinal axis of the molecule. The lowest energy structure of –menthol is a chair conformation where all three substituent groups are in equatorial positions see S1 Table. Now draw both of the chair conformations of this molecule and circle the more stable conformation equatiorial preference energies. B Neomenthol is a stereoisomer of menthol. The most stable conformation of cyclohexane is shown in Fig. You can see now the first year confirmation has less 13 die actual interaction between the age group as you can see in the structure and. Input Wedge And Hash Bonds As Appropriate To Clinch Chegg Com.
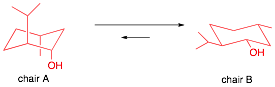 Source: ursula.chem.yale.edu
Source: ursula.chem.yale.edu
The chair form The structure of cis-14-dimethylcyclohexane is You can draw two flipped cyclohexane chairs. The first thing you need to know before drawing the ring-flip of a chair cyclohexane is the correct conformation of the carbon-chain and the orientation of each axial and equatorial group. Draw the following chair in the most stable conformation. The lowest energy structure of –menthol is a chair conformation where all three substituent groups are in equatorial positions see S1 Table. Draw both chair conformations for menthol a component of peppermint oil and its stereoisomer neomenthol. This is your first axial substituent. Final 01.
 Source: chegg.com
Source: chegg.com
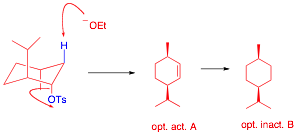
It is less stable than menthol but more stable. For neoisomenthol MD simulations showed two chair conformations. Draw both chair conformations chair and the chair flip and circle which is lower in energy most stable. It is less stable than menthol but more stable. Draw the following chair in the most stable conformation. In the given figure various possible chair conformations of 12-dimethylcyclohexane are drawn. Solved Neomenthol A Stereoisomer Of Menthol That Is Chegg Com.
 Source: chegg.com
Source: chegg.com
In order to get an overview of the potential energy surface we performed a relaxed two dimensional scan at the B3LYP3-21G level of theory by rotating the isopropyl. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to. Chair conformation of menthol is in menthol equotrial conformation are highly fused as all groups are equotrialHence it is more stable chair conformation of. This is consistent with previous computational. The chair form The structure of cis-14-dimethylcyclohexane is You can draw two flipped cyclohexane chairs. You can see now the first year confirmation has less 13 die actual interaction between the age group as you can see in the structure and. Solved The Structure Of Neomenthol Is Drawn Below On A Chegg Com.

B Neomenthol is a stereoisomer of menthol. Now draw both of the chair conformations of this molecule and circle the more stable conformation equatiorial preference energies. Neomenthol a stereoisomer of menthol that is isolated from various mint oils is shown below. The boat forms You can also draw two flipped boat conformations. Menthol is an organic compound made synthetically or obtained from the oils of corn mint peppermint or other mints. Draw the second chair conformation ring-flip-check this post if not sure. 2.
 Source: chegg.com
Source: chegg.com
Menthol is an organic compound made synthetically or obtained from the oils of corn mint peppermint or other mints. For neoisomenthol MD simulations showed two chair conformations. - - - - - - - - - - - - - - - - - - - - Question. It is less stable than menthol but more stable. This is consistent with previous computational. The chair form The structure of cis-14-dimethylcyclohexane is You can draw two flipped cyclohexane chairs. Draw Both Chair Conformations For Menthol A Component Of Chegg Com.
 Source: chegg.com
Source: chegg.com
For each chair conformer add the energy of all the groups on axial position. Draw both chair conformations for menthol a component of peppermint oil and its stereoisomer neomenthol. However in contrast to calculated energies and correlations between theoretical and experimental 13C chemical shifts the measured 3J H3H2 coupling of 63 Hz indicates an equally populated equi-librium of both conformers. In the first conformer we have two chlorines in axial positions so the total steric strain is. In these structures the hydroxyl group takes an axial orientation. The chair reference is just referring to the repeating zig-zag shape across the longitudinal axis of the molecule. Solved A Menthol Used To Flavor Various Foods And Tobacco Is Chegg Com.
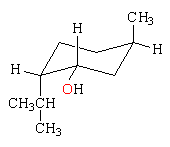 Source: askthenerd.com
Source: askthenerd.com
Organic Chemistry 2nd Edition Edit edition Solutions for Chapter 6 Problem 26AP. Neomenthol is the second most stable stereoisomer of 2-isopropyl-5-methylcyclohexanol. And now the stabilities. A sample of our newest video. Neomenthol a stereoisomer of menthol that is isolated from various mint oils is shown below. Ive seen chair conformation defined in that way before. Menthol And Neomenthol.










