chair conformation multiple choice questions The boat conformation is quite flexible. A11-dimethylcyclohexane Bcis-12-dimethylcyclohexane Ctrans-12-dimethylcyclohexane Dcis-13-dimethylcyclohexane.
Chair Conformation Multiple Choice Questions, Increases with chain length and. A 2 B 3 C 4 D 5 E 6 22 Arrange the following conformers of butane in order of energy lowest to highest. Each answer below shows example data from a table.
 Ring Flip Comparing The Stability Of Chair Conformations With Practice Problems Chemistry Steps From chemistrysteps.com
Ring Flip Comparing The Stability Of Chair Conformations With Practice Problems Chemistry Steps From chemistrysteps.com
Conformations are different arrangements of atoms that can be converted into one another by rotation about ___________. In order for the chair conformations to be of equal energy the two groups will 1 need to be the same and 2 need to be one equatorial and one axial. Gas constant R 83145 x 10-3 kJKmol 1. A 2 B 3 C 4 D 5 E 6 22 Arrange the following conformers of butane in order of energy lowest to highest. Chemistry questions and answers.
The chair conformation of cyclohexane is more stable than the boat conformation.
The most stable conformation for cyclohexane is the chair conformation. Engineering Chemistry Multiple Choice Questions on Conformations. Consider the stereoisomers cis-12-dimethylcyclohexane and trans-12-dimethylcyclohexane. Draw two different chair conformations of bromocyclohexane showing all hydrogen atoms. Answer the following questions and then press Submit to get your score.
Another Article :
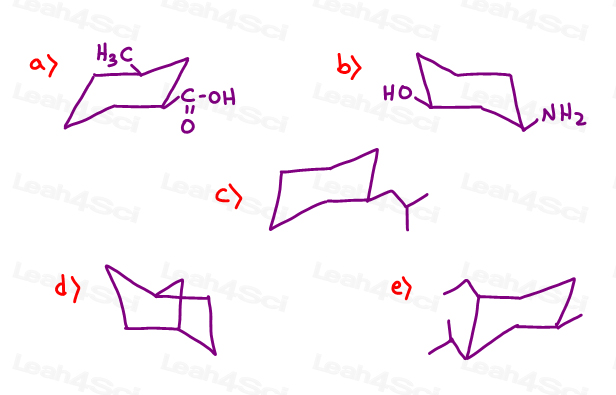 Source: leah4sci.com
Source: leah4sci.com
Write the two chair conformations of each of the following and in each part designate whichconformation would be more. The boat conformation is quite flexible. In order for the chair conformations to be of equal energy the two groups will 1 need to be the same and 2 need to be one equatorial and one axial. Chemistry questions and answers. Consider the stereoisomers cis-12-dimethylcyclohexane and trans-12-dimethylcyclohexane. Avogadros number 602 x 1023 moleculesmole. Cyclohexane Chair Conformations Organic Chemistry Practice Quiz.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Increases with chain length and. The most stable chair conformation of cis-13-cyclohexanediol has both hydroxyl groups in axial positions. For each of the following do two things. Determine if the highlighted atom will appear in the axial or equatorial position in the more stable chair conformation. Chemistry questions and answers. A11-dimethylcyclohexane Bcis-12-dimethylcyclohexane Ctrans-12-dimethylcyclohexane Dcis-13-dimethylcyclohexane. Ring Flip Of Chair Conformations With Practice Problems Chemistry Steps.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Gas constant R 83145 x 10-3 kJKmol 1. The most stable conformation for cyclohexane is the chair conformation. For each of the following do two things. So you wanna make sure you really know how to convert Fischer to Haworth and chair and back. The chair conformation of cyclohexane is more stable than the boat conformation. C d The less stable chair conformation of. Ring Flip Comparing The Stability Of Chair Conformations With Practice Problems Chemistry Steps.
 Source: pinterest.com
Source: pinterest.com
Identify whether the more stable stereoisomer is cis or trans. Increases with chain length and. Which answer is an example of the multivalue multicolumn problem. What are the products of. The chair conformation of cyclohexane is more stable than the boat conformation. Check out httppracticeproblemssolutions for more organic chemistry practice problems with links to video solutions. Construction Of A Recombinant Dna Molecule Recombinant Dna Dna Molecule Molecules.
 Source: pinterest.com
Source: pinterest.com
250 TOP MCQs on Conformations and Answers. A The methyl and bromine are cis and the chlorine and bromine are cis. IV A I B II C II D IV 14. Draw the most stable chair form for the more stable stereoisomer for the molecule B. Chemistry questions and answers. Select all the statements that correctly describe these two compounds. Newman Projections Practice Problems Organic Chemistry Organic Chemistry Study Newman.
 Source: pinterest.com
Source: pinterest.com
Question 13 1 Question 21-23 11. In either chair conformation of cis one methyl group will be axial and one will be equatorial. Engineering Chemistry Multiple Choice Questions on Conformations. Glucose galactose and mannose are among the most common carbohydrates in living cells. This example shows how to determine. Write the two chair conformations of each of the following and in each part designate whichconformation would be more. Kinematics Equation Physics Classroom Equations Physics.
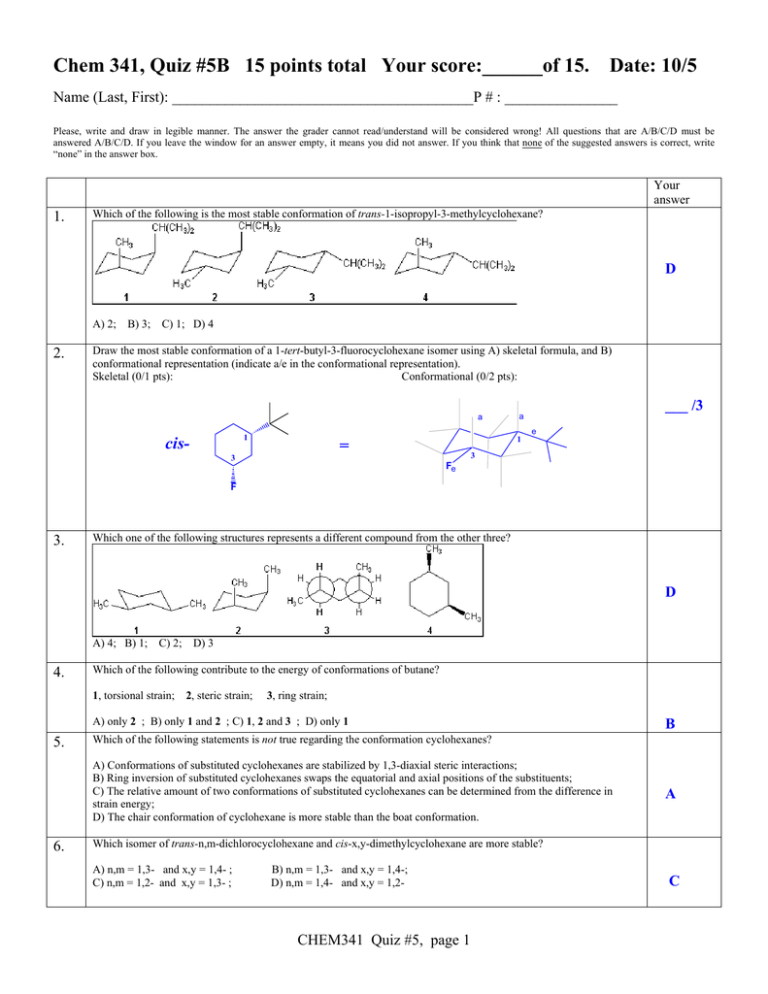 Source: studylib.net
Source: studylib.net
Write the two chair conformations of each of the following and in each part designate whichconformation would be more. A 2 B 3 C 4 D 5 E 6 22 Arrange the following conformers of butane in order of energy lowest to highest. Question 13 1 Question 21-23 11. Also six-membered cyclic forms pyranoses are also prevalent in nature so they are very important. All bonds to hydrogen atoms on adjacent carbon atoms are staggered and therefore torsional strain is minimized. The melting point of a lipid a. Chem 341 Quiz 5b 15 Points Total Your Score Of 15 Date.
 Source: leah4sci.com
Source: leah4sci.com
Draw the most stable chair form. View Test Prep - multiple choice questions_fall2006 final 1 from CHEM 8A at University of California Santa Cruz. 21 In the lowest energy chair conformation of cis-13-dimethylcyclohexane how many axial positions are occupied by hydrogen atoms. Also six-membered cyclic forms pyranoses are also prevalent in nature so they are very important. Would love to look at other chapter questions as well. Consider this chair conformation. Cyclohexane Chair Conformations Organic Chemistry Practice Quiz.
 Source: pinterest.com
Source: pinterest.com
The most stable conformation of trans-1-ethyl-3-fluorocyclohexane will be a chair conformation with the larger ethyl group equatorial and an axial F atom. Drawing the Chair Flipped Conformation. Draw the most stable chair form for the more stable stereoisomer for the molecule B. A The methyl and bromine are cis and the chlorine and bromine are cis. 250 TOP MCQs on Conformations and Answers. Gas constant R 83145 x 10-3 kJKmol 1. Pin On Ib Maths Resources.
 Source: study.com
Source: study.com
Write the two chair conformations of each of the following and in each part designate whichconformation would be more. Finally this is a very typical type of an exam question. Engineering Chemistry Multiple Choice Questions on Conformations. What are the products of. Glucose galactose and mannose are among the most common carbohydrates in living cells. Draw the most stable chair form for the more stable stereoisomer for the molecule B. Quiz Worksheet Cyclohexane Conformation Study Com.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Question 13 1 Question 21-23 11. Drawing the Chair Flipped Conformation. Identify each substituent as axial or equatorial. Multiple Choice Which of the following compounds can adopt a chair conformation in which there are no axial methyl groups. Although it is more stable the chair conformation is much more rigid than the boat conformation. Write the two chair conformations of each of the following and in each part designate whichconformation would be more. Drawing The Chair Conformation Of Cyclohexane Chemistry Steps.
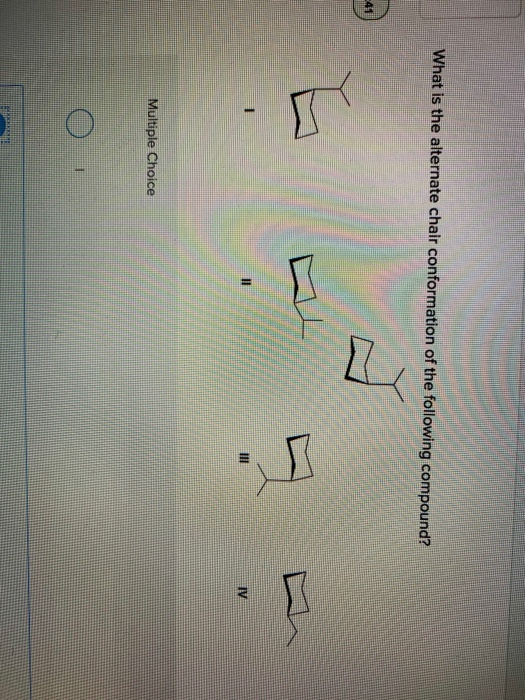 Source: chegg.com
Source: chegg.com
Identify each substituent as axial or equatorial. A I B II C III D IV 15. Question 14 8 Question 24-25 8. The chair form of cyclohexane is most stable and hence is the least energetic conformation. Although it is more stable the chair conformation is much more rigid than the boat conformation. An Introduction to Organic Chemistry- the Saturated Hydrocarbons. Solved What Is The Alternate Chair Conformation Of The Chegg Com.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Consider this chair conformation. Which of the following chair conformations represents trans-13-dimethylcyclohexane. The least strained form of any unsubstituted cycloalkane is the chair conformation of cyclohexane. Identify whether the more stable stereoisomer is cis or trans. Conformations are different arrangements of atoms that can be converted into one another by rotation about ___________. Gas constant R 83145 x 10-3 kJKmol 1. Ring Flip Comparing The Stability Of Chair Conformations With Practice Problems Chemistry Steps.
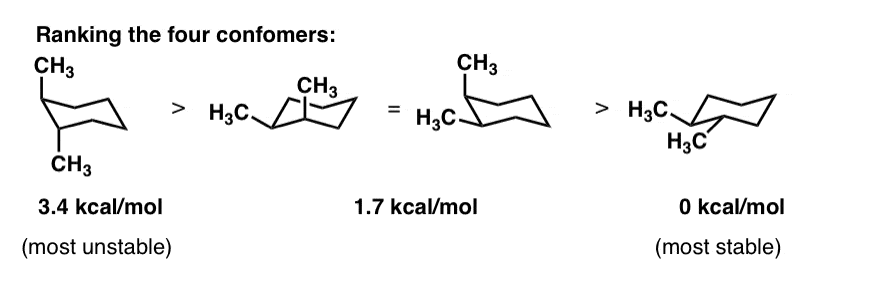 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Also six-membered cyclic forms pyranoses are also prevalent in nature so they are very important. The chair form of cyclohexane is most stable and hence is the least energetic conformation. Indicate the most stable conformation. Which of the following chair conformations represents trans-13-dimethylcyclohexane. Identify each substituent as axial or equatorial. B Draw a Newman projection of the most stable conformation of 1-bromopentane looking down the C_2-C_3 bond. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Test bank Questions and Answers of Chapter 10. A The methyl and bromine are cis and the chlorine and bromine are cis. A 2 B 3 C 4 D 5 E 6 22 Arrange the following conformers of butane in order of energy lowest to highest. The melting point of a lipid a. Test bank Questions and Answers of Chapter 10. Gas constant R 83145 x 10-3 kJKmol 1. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.










