chair conformation of cis 1 3 dimethylcyclohexane Draw the most stable conformation of cis-14-dimethylcyclohexane. IV A I B II C II D IV 14.
Chair Conformation Of Cis 1 3 Dimethylcyclohexane, Up To 10 Year Warranty. The two conformations are identical. A cis-12-dibromocyclohexane b trans-13-dimethylcyclohexane c cis-13-dichlorocyclohexane d cis-cyclohexane-13-diol.
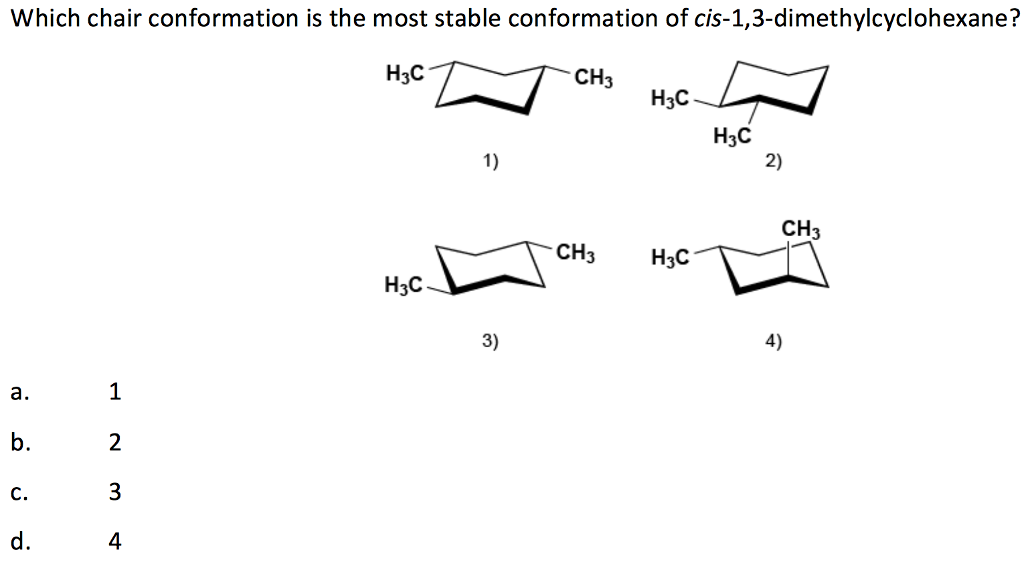 Solved Which Chair Conformation Is The Most Stable Chegg Com From chegg.com
Solved Which Chair Conformation Is The Most Stable Chegg Com From chegg.com
Another Article :
Which of the following chair conformations represents trans-13-dimethylcyclohexane. The E2 Reaction Cyclohexanes 1 Youtube. Ad Professional Efficient Delivery. Problem 20 Easy Difficulty. In case of cyclohexanes the overall molecule is not flat as often we sketch it on our notebooks.
Draw the chair conformations of.
In other words both conformations are meso. A Draw the two chair conformations of cis-13-dimethylcyclohexane and label all the positions as axial or equatorial. In the given figure various possible chair conformations of 12-dimethylcyclohexane are drawn. Consider Cis 1 3 Dimethylcyclohexane A Draw The Two Chair. 1 Answer 1 vote.
 Source: research.cm.utexas.edu
Source: research.cm.utexas.edu
Cyclohexane Conformational Analysis There are two possibilities that are cis or trans but the position of the methyl group on axial or equatorial bond on cyclohexane determines whether the compound is cis or trans. Draw chair conformation of cis-13-dimethylcyclohexane. Wide Range Of Furniture Mattresses Decor. Cis-13-dimethylcyclohexane another molecule with two asymmetric carbons is a mixture of conformational diastereomers. A Draw the two chair conformations of cis-13-dimethylcyclohexane and label all the positions as axial or equatorial. Organic Chemistry 9th Edition Edit edition Solutions for Chapter 5 Problem 73E.
 Source: research.cm.utexas.edu
Source: research.cm.utexas.edu
Cyclohexane Conformational Analysis The tert-butyl group equatorial and the methyl group axial. A Draw the two chair conformations of cis-13-dimethylcyclohexane and label all the positions as axial or equatorial. Cyclohexane Chair Conformations Organic Chemistry Practice Quiz. 15 points Write both chair conformations for both cis and trans isomers of 13-dimethylcyclohexane label them A B C and D. The most stable chair conformation of cis-1-tert-butyl-3-methylcyclohexane has. Answer Consider 1s 3r 1 3 Dimethylcycl Clutch Prep.
 Source: slideplayer.com
Source: slideplayer.com
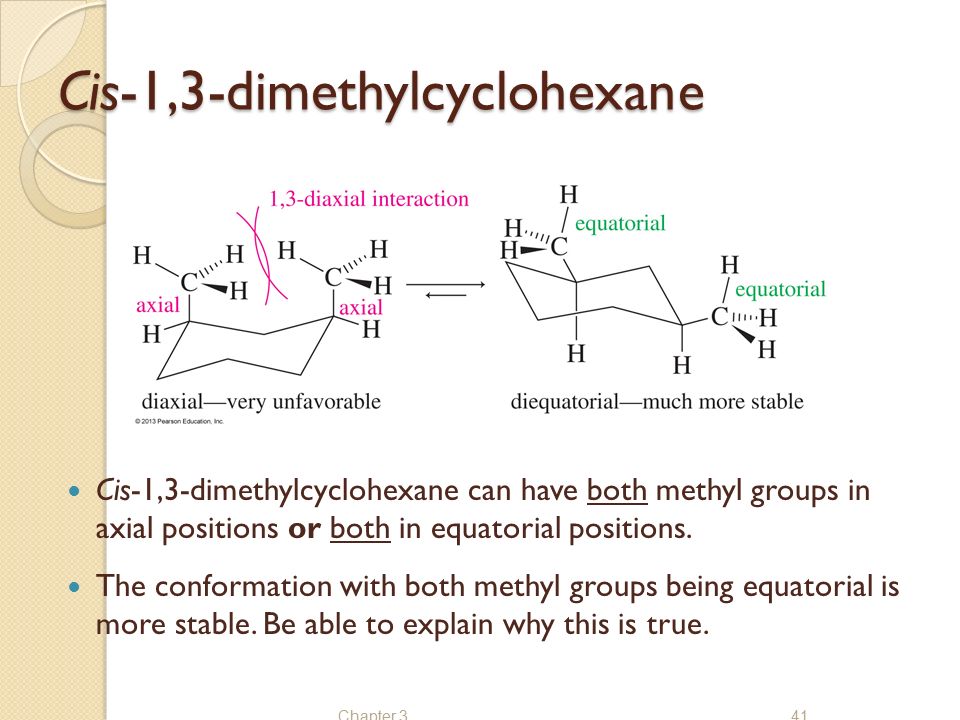
Structure And Stereochemistry Of Alkanes Ppt Video Online Download IV A I B II C II D IV 14. A Carbon and hydrogen B Carbon and water C Carbon dioxide and. Draw the most stable conformation of cis-14-dimethylcyclohexane. Answer Consider 1s 3r 1 3 Dimethylcycl Clutch Prep. The most stable chair conformation of cis-1-tert-butyl-3-methylcyclohexane has. A How many stereoisomers are there of cis-13-dimethylcyclohexane and how many of trans-13-dimethylcyclohexane.
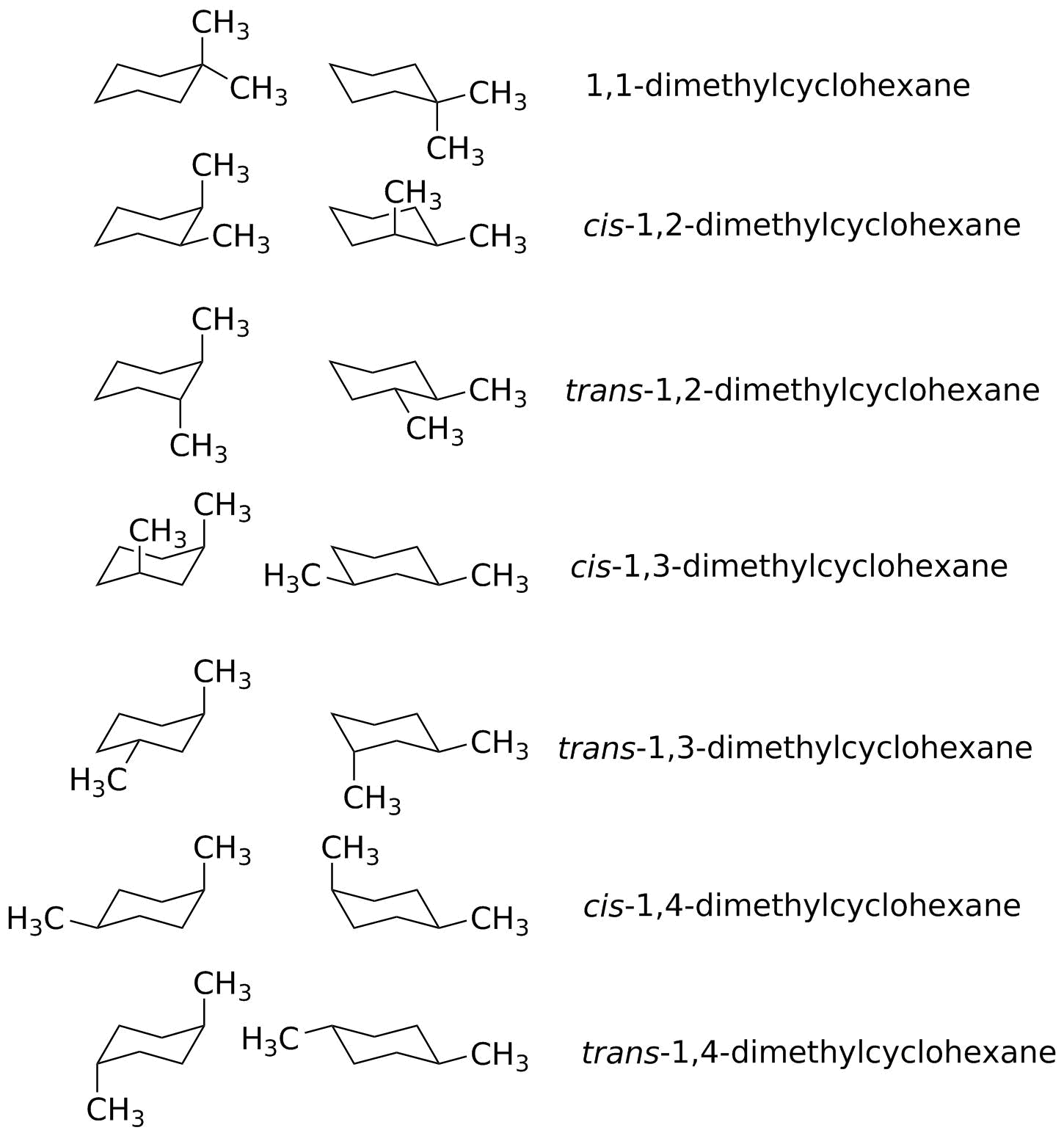 Source: sites.science.oregonstate.edu
Source: sites.science.oregonstate.edu
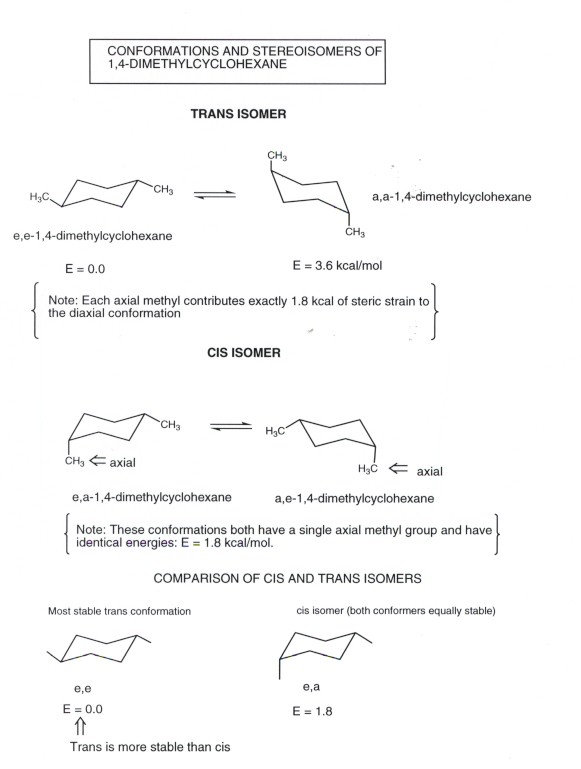
Chapter 4 Worked Problem 1 Conformation of cis-13-dimethylcyclohexane disubstituted - Can have both methyl groups in axial positions or both in equatorial positions. Yet even though each conformation has two asymmetric carbons neither conformation is chi-ral because each has an internal plane of symmetry. The two chair conformations are equal in energy. If they occupy the axial positions then it will be the highest energy chair conformation because of the unfavorable 13. For cis-13-dimethylcyclohexane the lowest energy chair conformation implies that the methyl groups of 1 and 3 carbons of the cyclohexane ring must occupy the equatorial positions. There are one chair conformation and two boat conformations of cis-14-dimethylcyclohexane.
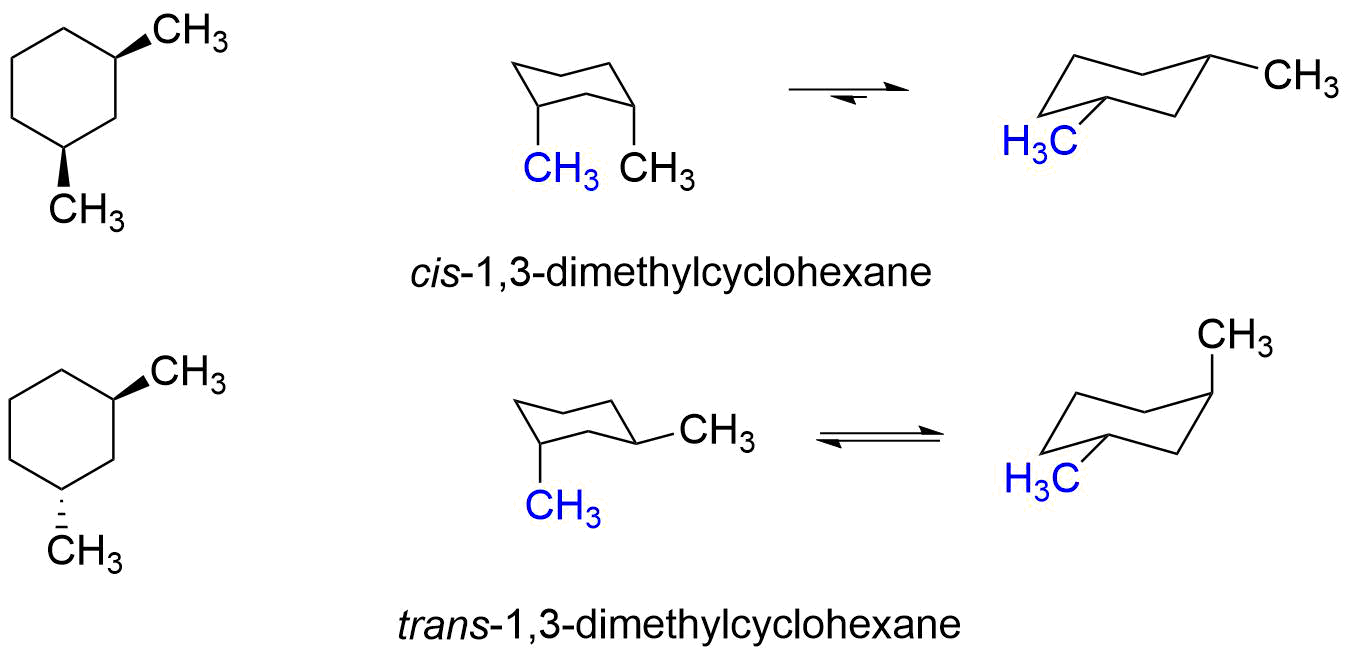 Source: chem.libretexts.org
Source: chem.libretexts.org
3 9 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts 1 Answer 1 vote. Conformation of cis-13-dimethylcyclohexane disubstituted - Can have both methyl groups in axial positions or both in equatorial positions. Ad Professional Efficient Delivery. The trans-13-dimethylcyclohexane isomer on the other hand has one methyl axial in both ring-flip conformers so that it is less stable than the cis isomer by 18 kcalmol. Organic Chemistry 9th Edition Edit edition Solutions for Chapter 5 Problem 73E. Cis-13-dimethylcyclohexane is thus a.

Solved Which Is More Stable Cis 1 3 Dimethylcyclohexane Or Trans 1 3 Dimethylcyclohexane Draw A cis-12-dibromocyclohexane b trans-13-dimethylcyclohexane c cis-13-dichlorocyclohexane d cis-cyclohexane-13-diol. 100 Day Free Returns. For cis-1 3-dimethylcyclohexane which two chair conformations are in equilibrium. B Label the higher-energy conformation and the lower-energy conformation. A Indicate by a label whether each methyl group is axial or equatorial. B For which isomers are the alternative chair conformations of equal stability.
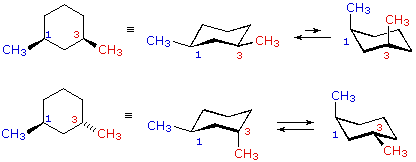 Source: chem.libretexts.org
Source: chem.libretexts.org
4 10 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts Like in given figure no. If they occupy the axial positions then it will be the highest energy chair conformation because of the unfavorable 13. Make your chair structures clear and accurate and identify axial methyls by circling them. A Carbon and hydrogen B Carbon and water C Carbon dioxide and. In the lowest energy chair conformation of cis-13-dimethylcyclohexane how many axial positions are occupied by hydrogen atoms. For cis-1 3-dimethylcyclohexane which two chair conformations are in equilibrium.
 Source: onlineorganicchemistrytutor.com
Source: onlineorganicchemistrytutor.com
Possible Chair Conformations Of 1 2 Dimethylcyclohexane In other words both conformations are meso. Oneclass Construct The Most Stable Isomer And Conformation Of 1 3. In real sense the cyclohexanes adopts two conformations. Which of the following chair conformations represents trans-13-dimethylcyclohexane. Draw the alternative chair conformation for the cis and trans isomers of 12-dimethylcyclohexane 13-dimethylcyclohexane and 14-dimethylcyclohexane. For cis-13-dimethylcyclohexane one chair conformation has both methyl groups in axial positions creating 13-diaxial interactions.
 Source: clutchprep.com
Source: clutchprep.com
For Cis 1 3 Dimethylcyclohexane Which Two Clutch Prep In the lowest energy chair conformation of cis-13-dimethylcyclohexane how many axial positions are occupied by hydrogen atoms. The two conformations are identical. In the lowest energy chair conformation of cis-13-dimethylcyclohexane how many axial positions are occupied by hydrogen atoms. Which of the following is the most stable conformation of cis-1-ethyl-3- isopropylcyclohexane. For cis-13-dimethylcyclohexane one chair conformation has both methyl groups in axial positions creating 13-diaxial interactions. Answered May 15 2019 by Sweety01 701k points selected May 17 2019.
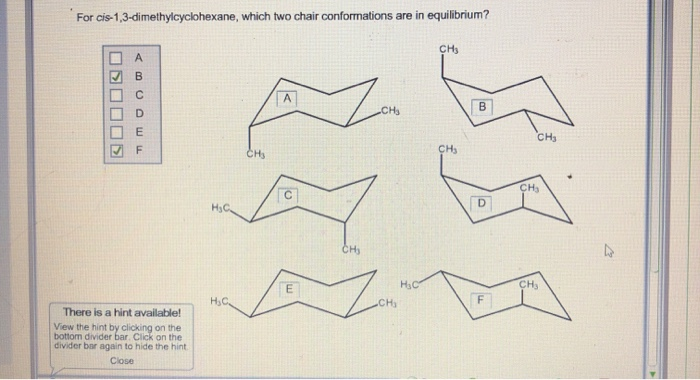 Source: chegg.com
Source: chegg.com
Solved For Cis 1 3 Dimethylcyclohexane Which Two Chair Chegg Com The tert-butyl group equatorial and the methyl group axial. Wide Range Of Furniture Mattresses Decor. B Label the higher-energy conformation and the lower-energy conformation. There is only one chair conformation of cis-14-dimethylcyclohexane. Draw chair conformation of cis-13-dimethylcyclohexane. The two conformations are identical.
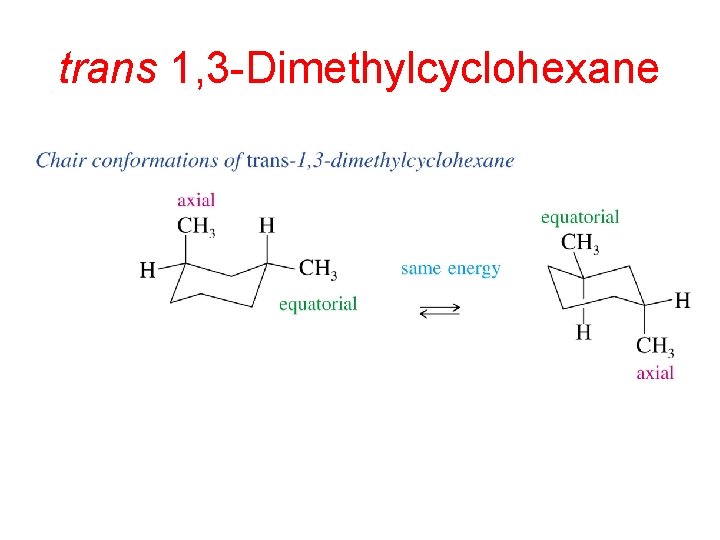 Source: slidetodoc.com
Source: slidetodoc.com
Conformational Analysis Newman Projections Ring Strain Cyclohexane Conformations A Draw the two chair conformations of cis-13-dimethylcyclohexane and label all the positions as axial or equatorial. The two conformations are identical. A Draw the two chair conformations of cis-13-dimethylcyclohexane and label all the positions as axial or equatorial. Answer Consider 1s 3r 1 3 Dimethylcycl Clutch Prep. - when neither chair conformation allows both. Yet even though each conformation has two asymmetric carbons neither conformation is chi-ral because each has an internal plane of symmetry.
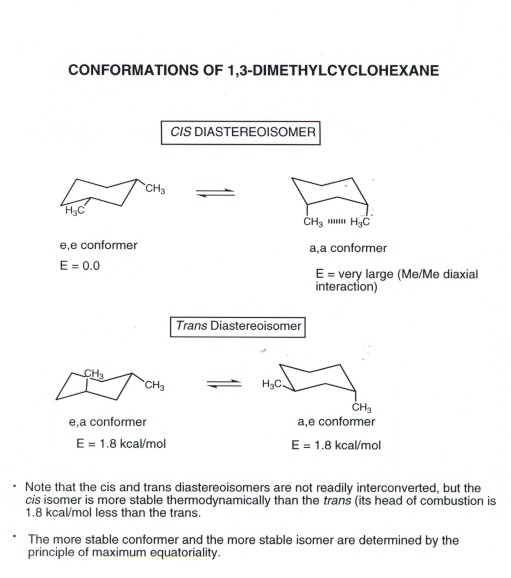 Source: research.cm.utexas.edu
Source: research.cm.utexas.edu
Cyclohexane Conformational Analysis 100 Day Free Returns. There are one chair conformation and two boat conformations of cis-14-dimethylcyclohexane. For cis-13-dimethylcyclohexane one chair conformation has both methyl groups in axial positions creating 13-diaxial interactions. A cis-12-dibromocyclohexane b trans-13-dimethylcyclohexane c cis-13-dichlorocyclohexane d cis-cyclohexane-13-diol. 15 points Write both chair conformations for both cis and trans isomers of 13-dimethylcyclohexane label them A B C and D. Draw the most stable conformation of cis-14-dimethylcyclohexane.
 Source: chegg.com
Source: chegg.com
Solved Which Chair Conformation Is The Most Stable Chegg Com A How many stereoisomers are there of cis-13-dimethylcyclohexane and how many of trans-13-dimethylcyclohexane. Which of the following is the most stable conformation of cis-1-ethyl-3- isopropylcyclohexane. Answered May 15 2019 by Sweety01 701k points selected May 17 2019. Yet even though each conformation has two asymmetric carbons neither conformation is chi-ral because each has an internal plane of symmetry. Draw chair conformation of cis-13-dimethylcyclohexane. There is only one chair conformation of cis-14-dimethylcyclohexane.
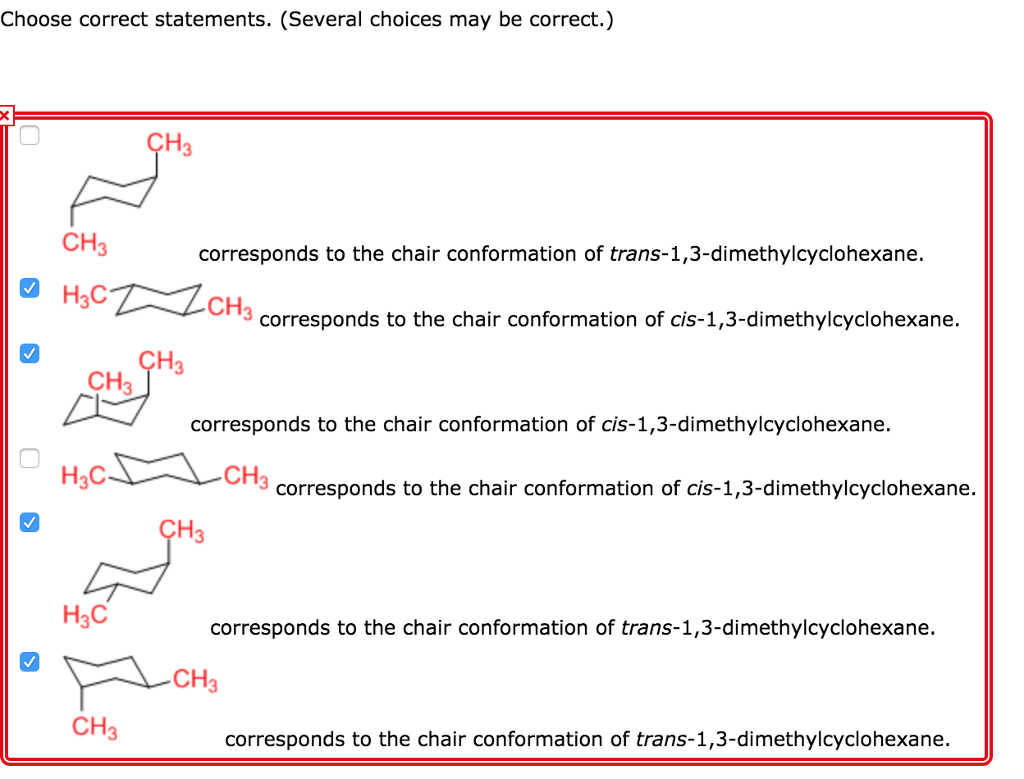 Source: chegg.com
Source: chegg.com
Solved For Each Of Two Stereoisomers Of Chegg Com - when neither chair conformation allows both. Draw the chair conformations of. 100 Day Free Returns. Cis-13-dimethylcyclohexane is thus a. In other words both conformations are meso. The tert-butyl group equatorial and the methyl group axial.
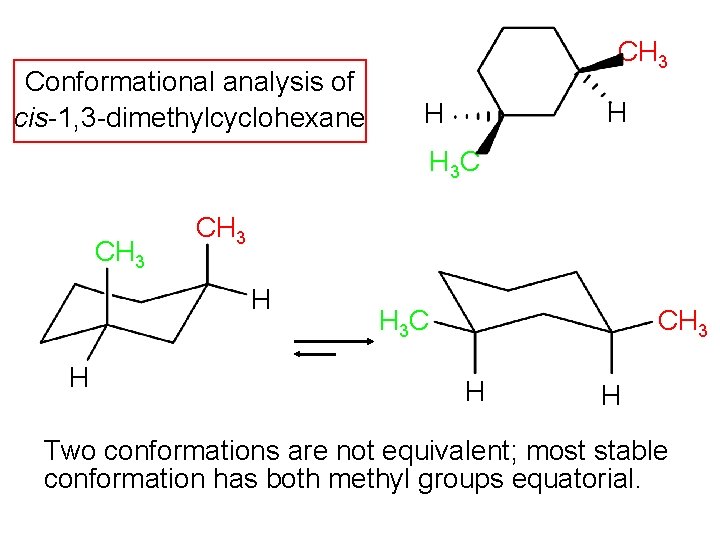 Source: slidetodoc.com
Source: slidetodoc.com
Chapter 3 Alkanes And Cycloalkanes Conformations And Cistrans Organic Chemistry 9th Edition Edit edition Solutions for Chapter 5 Problem 73E. 1 Answer 1 vote. The tert-butyl group axial and the methyl group equatorial. Make your chair structures clear and accurate and identify axial methyls by circling them. The two conformations are identical. There is only one chair conformation of cis-14-dimethylcyclohexane.
 Source: quizlet.com
Source: quizlet.com
Chapter 3 Cyclohexane Conformations Flashcards Quizlet Conformation of cis-13-dimethylcyclohexane disubstituted - Can have both methyl groups in axial positions or both in equatorial positions. For cis-13-dimethylcyclohexane one chair conformation has both methyl groups in axial positions creating 13-diaxial interactions. If they occupy the axial positions then it will be the highest energy chair conformation because of the unfavorable 13. The tert-butyl group equatorial and the methyl group axial. A boat conformation and a chair. In the lowest energy chair conformation of cis-13-dimethylcyclohexane how many axial positions are occupied by hydrogen atoms.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy Cis-13-dimethylcyclohexane is thus a. A similar conformational analysis can be made for the cis and trans stereoisomers of 13-dimethylcyclohexane. Cyclohexane Chair Conformations Organic Chemistry Practice Quiz. If they occupy the axial positions then it will be the highest energy chair conformation because of the unfavorable 13. In the given figure various possible chair conformations of 12-dimethylcyclohexane are drawn. For cis-1 3-dimethylcyclohexane which two chair conformations are in equilibrium.
 Source: oneclass.com
Source: oneclass.com
Oneclass For Cis 1 3 Dimethylcyclohexane Which Two Chair Conformations Are In Equilibrium The tert-butyl group axial and the methyl group equatorial. B For which isomers are the alternative chair conformations of equal stability. In other words both conformations are meso. 1 Answer 1 vote. Which of the following chair conformations represents trans-13-dimethylcyclohexane. Cis-13-dimethylcyclohexane another molecule with two asymmetric carbons is a mixture of conformational diastereomers.
Please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title chair conformation of cis 1 3 dimethylcyclohexane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.











