chair conformation lowest energy The single bond is active default GH. The chair is the lowest energy conformation for cyclohexane.
Chair Conformation Lowest Energy, Number the ring and draw any chair conformation of the compound. If they occupy the axial positions then it will be the highest energy chair conformation because of. The boat conformation suffers from torsional strain making it less stable higher in energy than the chair.

Another Article :
In the first conformer we have two chlorines in axial positions so the total steric strain is. Since the chair conformation has the lowest potential energy it is the most relevant to the conformation of cyclohexane. If they occupy the axial positions then it will be the highest energy chair conformation because of. Show transcribed image text Expert Answer. Draw cis-1-ethyl-3-methylcyclohexane in its lowest energy conformation.
The other conformations are known as the twist and boat forms.
We review their content and. An alternate conformation for a six-membered ring is called the boat. Lowest energy form is the most important. The definition of chair conformation is the lowest energy conformation for cyclohexane in which all bond angles are fairly close to 1095 and all hydrogen atoms are staggered. Another conformation which is important in any conformational analysis is the transition state or maximum energy conformation on the rotational pathFor cyclohexane this is the so-called half-chair conformation in which now 5 carbons are co-planar and only one is puckered out of the plane.
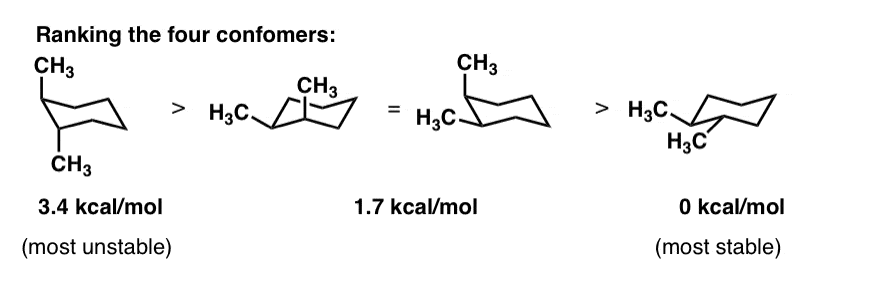 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy Explaining how A-Values are related to cyclohexane flip energy. The boat conformation suffers from torsional strain making it less stable higher in energy than the chair. Energy values taken from Organic Chemistry 5th edition by John McMurry p. Show transcribed image text Expert Answer. In the boat conformation two of the substituents those on the bow and the stern if you will are brought close enough to each other to cause. Notice that half of the hydrogen atoms have a different chemical environment.
 Source: clutchprep.com
Source: clutchprep.com
Draw The Lowest Energy Chair Conformation Clutch Prep 136 Conformer B for 1R-33-dichlorocyclohexanol is lower energy and more stable than conformer A. For each chair conformer add the energy of all the groups on axial position. Draw the second chair conformation ring-flip-check this post if not sure. The chair conformation is the most stable conformation of cyclohexane. The depiction below on the left has the red-colored hydrogen atoms in what is termed axial conformation. Cyclohexanes undergo fairly facile conformational exchange where axial substituents are exchanged for equatorial substituents and vice versa.
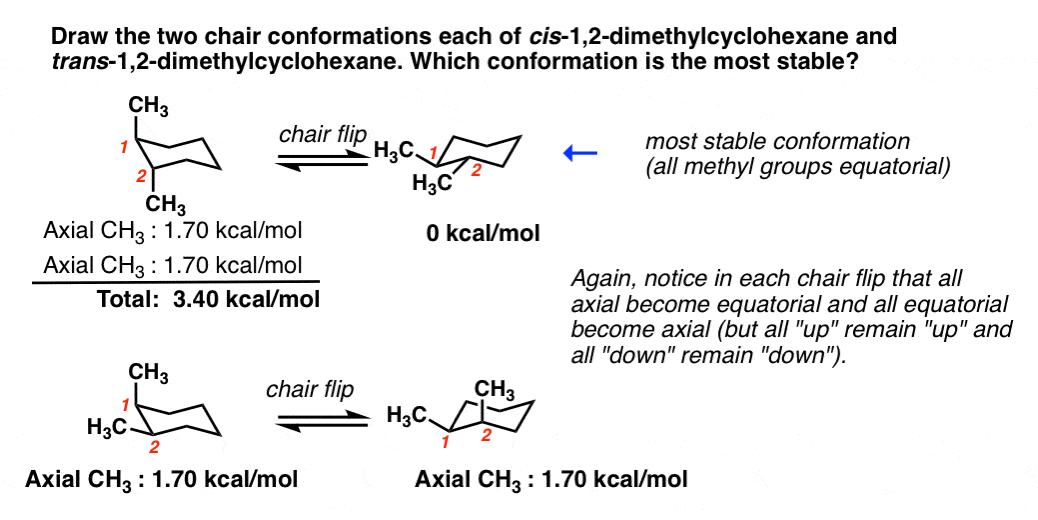 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy Lowest energy form is the most important. Show transcribed image text Expert Answer. I take it you are talking about substituted and disubstituted cyclohexanes. The structure above is the most stable structure of trans-1-ethyl-2-isopropylcyclohexane hence it has the lowest energy. The boat conformation suffers from torsional strain making it less stable higher in energy than the chair. The depiction below on the left has the red-colored hydrogen atoms in what is termed axial conformation.
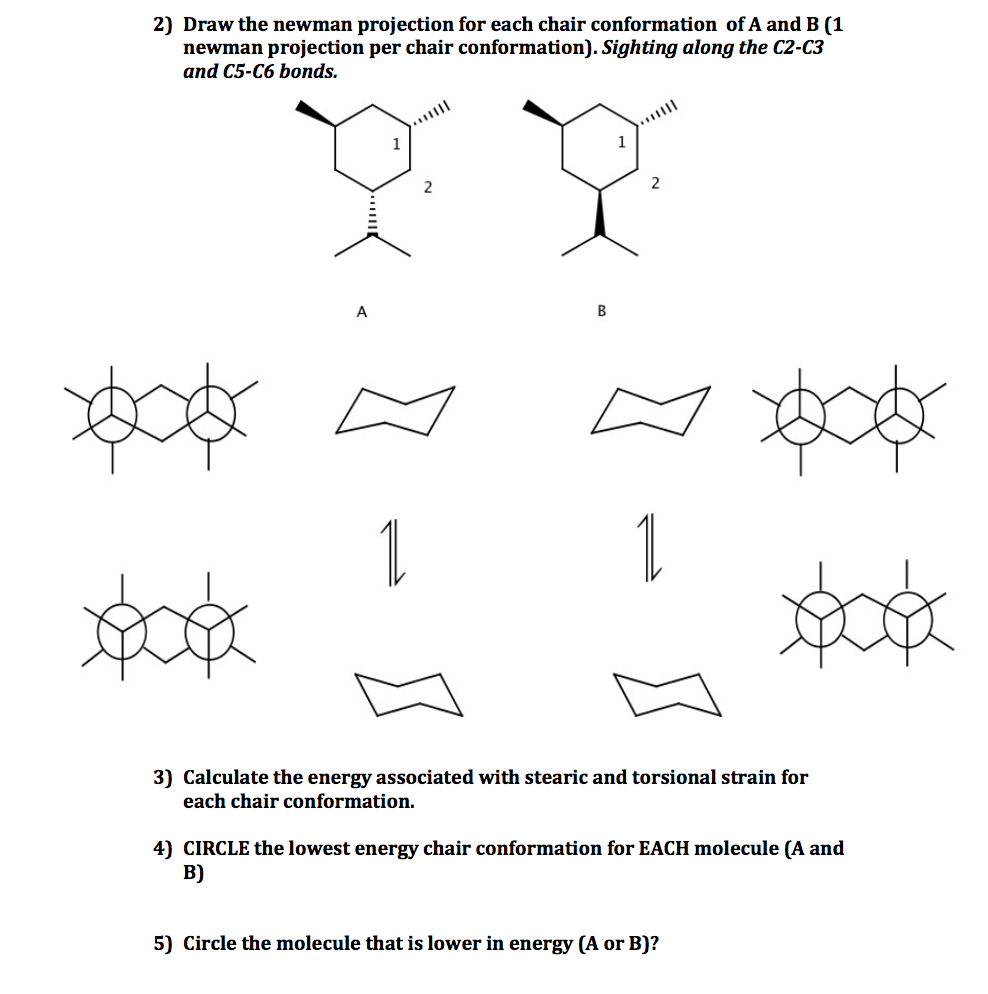
Solved Draw The Lowest And Highest Energy Conformations For Chegg Com So the relative stabilities are. Draw the second chair conformation ring-flip-check this post if not sure. The depiction below on the left has the red-colored hydrogen atoms in what is termed axial conformation. The chair conformation that you have drawn 4 C 1 is likely to be the most stable one as it minimizes the number of heavy axial groupsTo determine the chair conformation of a hexose it is generally easiest to draw it and compare it with β-D-glucose where all heavy groups are equatorial and the conformation is 4 C 1If the number of heavy axial groups becomes smaller when the conformation. Show transcribed image text Expert Answer. If the tert-butyl group is placed in the axial bond then the chair has the highest energy or the least stable conformation.
 Source: youtube.com
Source: youtube.com
Which Is The Lowest Energy Conformation Of Butane Youtube Chair twist boat boat half-chair. Make sure its the appropriate chair including any heterostoms. The following guidelines can be used to determine the percent distribution of two chair conformers using the Gibbs free energy equation and some simple algebra. Notice that half of the hydrogen atoms have a different chemical environment. Is lowest energy conformation most stable. In the boat conformation two of the substituents those on the bow and the stern if you will are brought close enough to each other to cause.
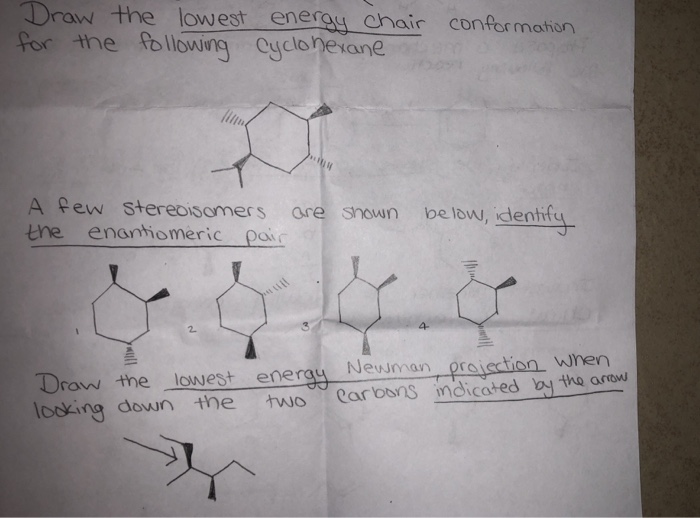
1 Draw The Lowest Energy Energy Chair Conformation Chegg Com Energy values taken from Organic Chemistry 5th edition by John McMurry p. Chair twist boat boat half-chair. 21 In the lowest energy chair conformation of cis-13-dimethylcyclohexane how many axial positions are occupied by hydrogen atoms. Run a molecular dynamics simulation in order to get a sense of the flexibility of the cyclohexane ring. Choose a chair from the Templates toolbar at the bottom. In the boat conformation two of the substituents those on the bow and the stern if you will are brought close enough to each other to cause.
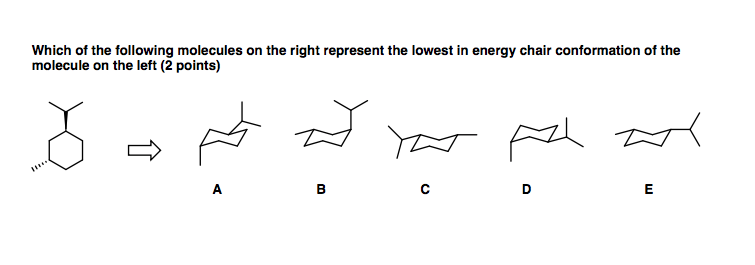 Source: chegg.com
Source: chegg.com
Solved Which Of The Following Molecules On The Right Chegg Com The lower energy chair conformation contains two axial methyl groups. The following guidelines can be used to determine the percent distribution of two chair conformers using the Gibbs free energy equation and some simple algebra. And models will always be permitted in examinations. A 2 B 3 C 4 D 5 E 6 22 Arrange the following conformers of butane in order of energy lowest to highest. Carefully study this molecule by rotating it on your screen. The lowest energy conformation it attains with its tetrahedral carbon atoms simulates a chair.
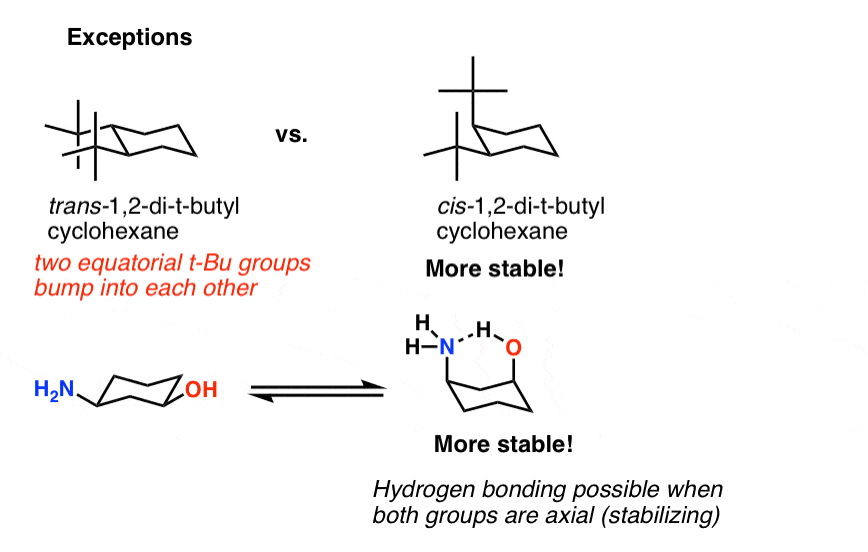 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy The chair conformation that you have drawn 4 C 1 is likely to be the most stable one as it minimizes the number of heavy axial groupsTo determine the chair conformation of a hexose it is generally easiest to draw it and compare it with β-D-glucose where all heavy groups are equatorial and the conformation is 4 C 1If the number of heavy axial groups becomes smaller when the conformation. Is lowest energy conformation most stable. 21 In the lowest energy chair conformation of cis-13-dimethylcyclohexane how many axial positions are occupied by hydrogen atoms. Draw cis-1-ethyl-3-methylcyclohexane in its lowest energy conformation. The boat conformation suffers from torsional strain making it less stable higher in energy than the chair. The chair conformation that you have drawn 4 C 1 is likely to be the most stable one as it minimizes the number of heavy axial groupsTo determine the chair conformation of a hexose it is generally easiest to draw it and compare it with β-D-glucose where all heavy groups are equatorial and the conformation is 4 C 1If the number of heavy axial groups becomes smaller when the conformation.
 Source: research.cm.utexas.edu
Source: research.cm.utexas.edu
Cyclohexane Conformational Analysis Rank these two low energy conformers as more or less stable. Energy values taken from Organic Chemistry 5th edition by John McMurry p. For each chair conformer add the energy of all the groups on axial position. Draw the lowest energy chair conformer for each structure A and B. Explaining how A-Values are related to cyclohexane flip energy. And models will always be permitted in examinations.
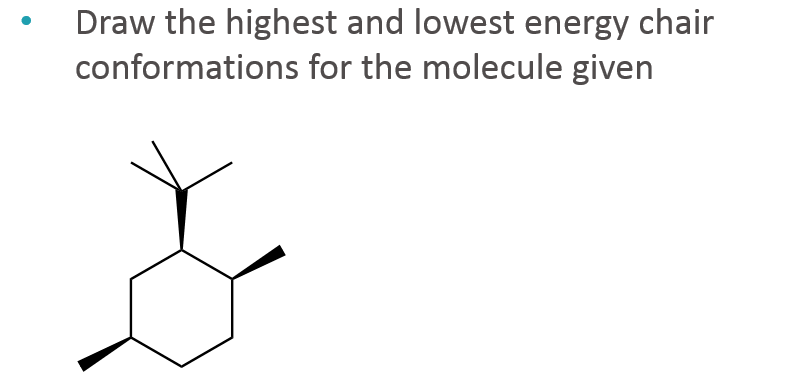 Source: chegg.com
Source: chegg.com
Solved Draw The Highest And Lowest Energy Chair Chegg Com Draw the second chair conformation ring-flip-check this post if not sure. An alternate conformation for a six-membered ring is called the boat. The lower energy chair conformation contains two axial methyl groups. Draw cis-1-ethyl-4-methylcyclohexane in its lowest energy conformation 10 Part A Draw etyl-4-methylcyclohexane in its lowest energy conformation 1. This chair conformation is the lowest energy conformation for cyclohexane and other six-membered rings. Draw the lowest energy chair conformer for each structure A and B.
![]() Source: study.com
Source: study.com
Draw Cis 1 Ethyl 3 Methylcyclohexane In Its Lowest Energy Conformation Study Com The higher energy chair conformation contains two axial methyl groups. First we have to introduce the concept of an A-value which is simply the energy difference between the equatorial most stable and axial least stable positions. In this conformation the isopropyl group prefers to be attached at the. Number the ring and draw any chair conformation of the compound. The chair conformation that you have drawn 4 C 1 is likely to be the most stable one as it minimizes the number of heavy axial groupsTo determine the chair conformation of a hexose it is generally easiest to draw it and compare it with β-D-glucose where all heavy groups are equatorial and the conformation is 4 C 1If the number of heavy axial groups becomes smaller when the conformation. Energy values taken from Organic Chemistry 5th edition by John McMurry p.

Solved Which Of The Conformers Is The Lowest Energy Chair Chegg Com The following guidelines can be used to determine the percent distribution of two chair conformers using the Gibbs free energy equation and some simple algebra. First we have to introduce the concept of an A-value which is simply the energy difference between the equatorial most stable and axial least stable positions. The higher energy chair conformation contains two axial methyl groups. The staggered conformation of ethane is. The chair conformation that you have drawn 4 C 1 is likely to be the most stable one as it minimizes the number of heavy axial groupsTo determine the chair conformation of a hexose it is generally easiest to draw it and compare it with β-D-glucose where all heavy groups are equatorial and the conformation is 4 C 1If the number of heavy axial groups becomes smaller when the conformation. Cyclohexanes undergo fairly facile conformational exchange where axial substituents are exchanged for equatorial substituents and vice versa.
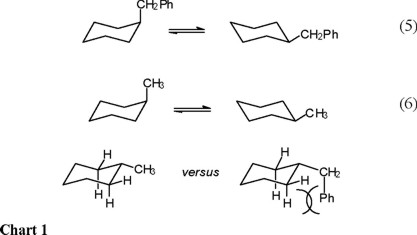 Source: scielo.org.mx
Source: scielo.org.mx
Enthalpic And Entropic Contributions To The Conformational Free Energy Differences In Monosubstituted Cyclohexanes The boat conformation is again a puckered structure that allows tetrahedral bond angles. Make sure its the appropriate chair including any heterostoms. On careful examination of a chair conformation of cyclohexane we find that the twelve hydrogens are not structurally equivalent. Which of the following is the lowest energy chair conformation of the following cyclohexane. The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. This too is almost free of angle strain but in contrast has torsional strain associated with eclipsed bonds at the four of the C atoms that form the side of the boat.
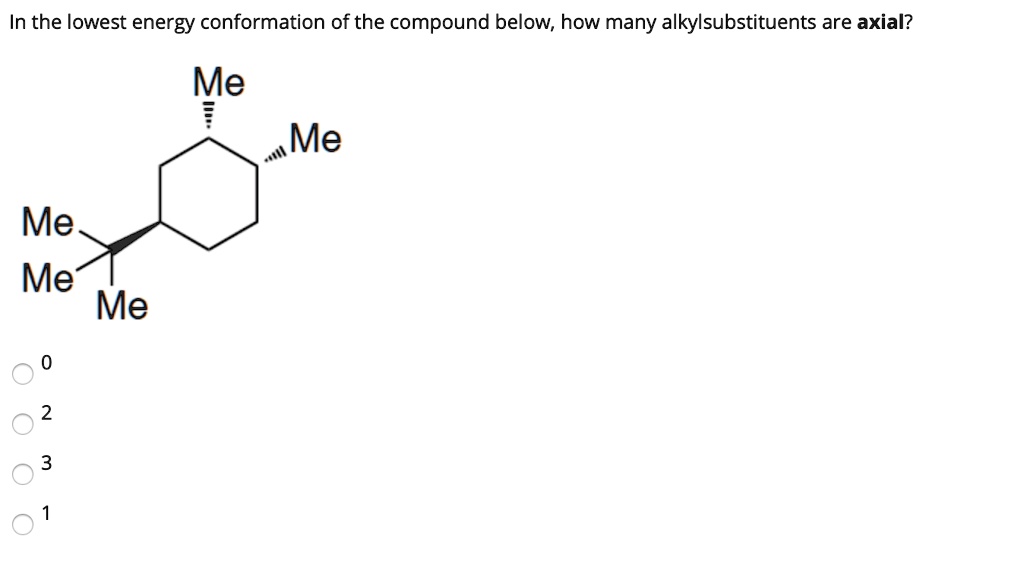 Source: numerade.com
Source: numerade.com
Solved In The Lowest Energy Conformation Of The Compound Below How Many Alkylsubstituents Are Axial Me Me Me Me Me Always place the largesthighest priority group in the equatorial position. Draw the following chair in the most stable conformation. A 2 B 3 C 4 D 5 E 6 22 Arrange the following conformers of butane in order of energy lowest to highest. The Chair Conformation - a closer look. In the first conformer we have two chlorines in axial positions so the total steric strain is. In the boat conformation two of the substituents those on the bow and the stern if you will are brought close enough to each other to cause.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Ring Flip Comparing The Stability Of Chair Conformations With Practice Problems Chemistry Steps A 2 B 3 C 4 D 5 E 6 22 Arrange the following conformers of butane in order of energy lowest to highest. A The two chair conformations are equal in energy. On careful examination of a chair conformation of cyclohexane we find that the twelve hydrogens are not structurally equivalent. There is no substitute for the use of models. Who are the experts. If the tert-butyl group is placed in the axial bond then the chair has the highest energy or the least stable conformation.
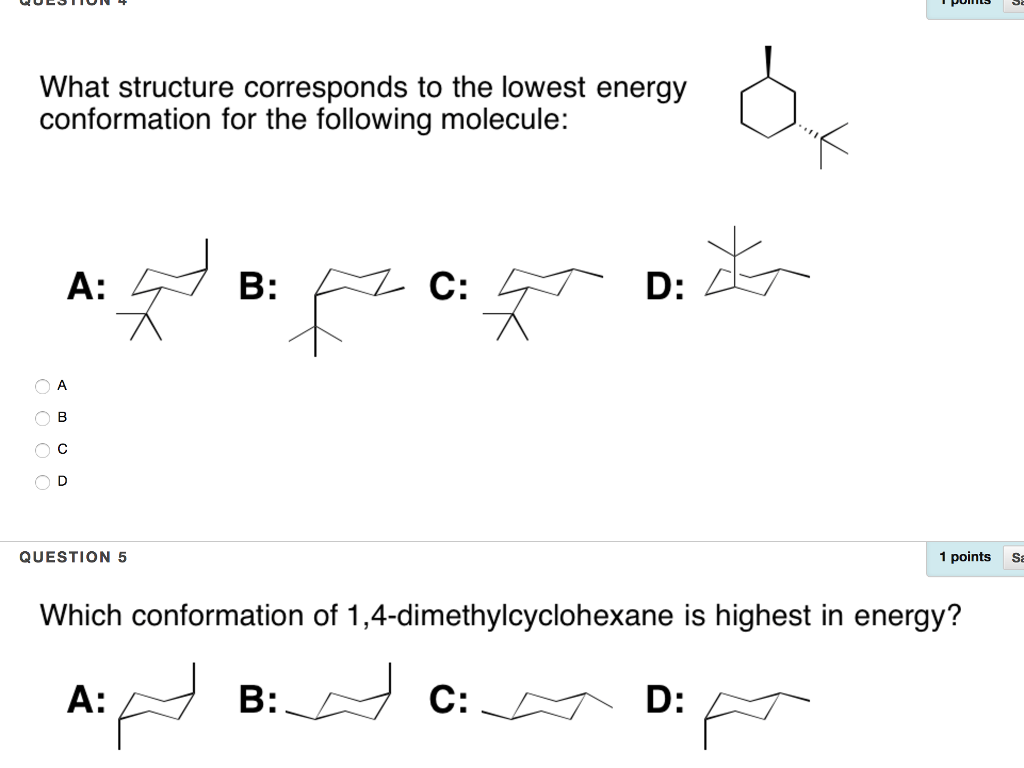 Source: chegg.com
Source: chegg.com
Solved What Structure Corresponds To The Lowest Energy A Chegg Com The structure above is the most stable structure of trans-1-ethyl-2-isopropylcyclohexane hence it has the lowest energy. Rank these two low energy conformers as more or less stable. Energy values taken from Organic Chemistry 5th edition by John McMurry p. An alternate conformation for a six-membered ring is called the boat. For each chair conformer add the energy of all the groups on axial position. The twist boat has a lower energy than the boat.
 Source: clutchprep.com
Source: clutchprep.com
Solution Draw The Lowest Energy Chair Co Organic Chem On careful examination of a chair conformation of cyclohexane we find that the twelve hydrogens are not structurally equivalent. The definition of chair conformation is the lowest energy conformation for cyclohexane in which all bond angles are fairly close to 1095 and all hydrogen atoms are staggered. The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. Chair twist boat boat half-chair. For cis-13-dimethylcyclohexane the lowest energy chair conformation implies that the methyl groups of 1 and 3 carbons of the cyclohexane ring must occupy the equatorial positions. 136 Conformer B for 1R-33-dichlorocyclohexanol is lower energy and more stable than conformer A.
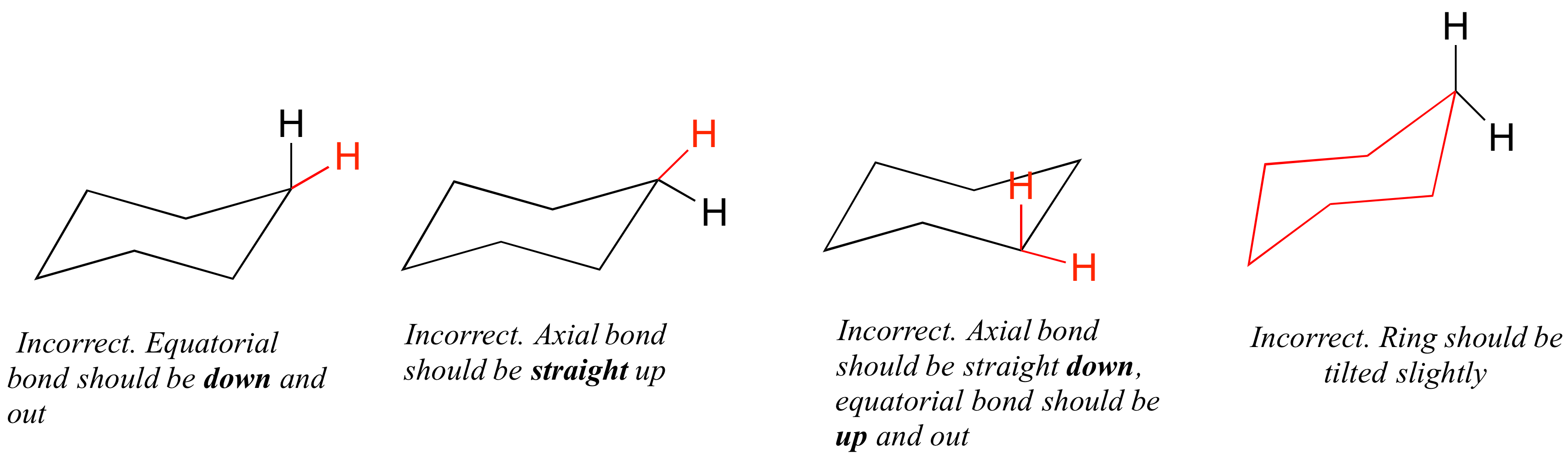 Source: chem.libretexts.org
Source: chem.libretexts.org
3 2 Conformations Of Cyclic Organic Molecules Chemistry Libretexts The chair conformation is considered to be the lowest energy conformation because it lacks both angle strain and torsional strainThis means that the chair conformation is the structure. In this conformation the isopropyl group prefers to be attached at the. The chair conformation is the most stable conformation of cyclohexane. Chair twist boat boat half-chair. The higher energy chair conformation contains two axial methyl groups. Which conformation has highest potential energy.
Please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title chair conformation lowest energy by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.











