chair conformation geometry Without flipping the chair simply rotate the molecule again until it looks like the chair conformation on the right. The first form of cyclohexane you build is the chair form.
Chair Conformation Geometry, The Chair Conformation The stability data in Table 71 require that the bond angles in cyclohexane must be essentially the same as the bond angles in an alkanevery close to the ideal 1095 tetrahedral angle. Building Chair Cyclohexane. Most commonly encountered in the chair conformation of cyclohexane.
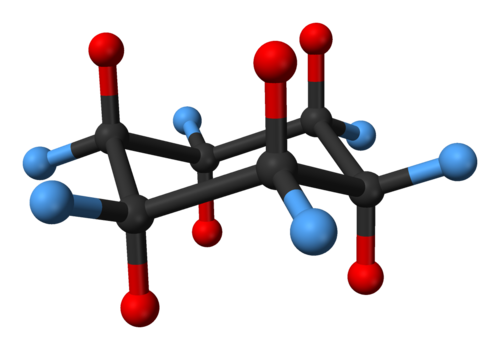
 Stereoisomeric Conformations Different Perspectives Achiral Stereoisomers And Meso Compounds Organic Chemistry Solutions From organicchemistrysolutions.com
Stereoisomeric Conformations Different Perspectives Achiral Stereoisomers And Meso Compounds Organic Chemistry Solutions From organicchemistrysolutions.com
Another Article :
Energy associated with a system due to its geometry. The CC stretching frequencies of the chair form are found to be higher than those of the planar ones. In fact cyclohexane take the chair conformation to achieve a bond angle similar to the tetrahedral geometry to eliminate angular strain. Lets get some practice drawing it chair confirmations one way to do it is to start by drawing two parallel lines that are offset from each others let me go ahead and show you what I mean so heres heres one line and then here is another line theyre parallel to each other but theyre offset a little bit next were going to draw two horizontal dotted lines so the top horizontal dotted line is going to be on level with that top. What is the most stable chair conformation.
However keep in mind that the geometry of the cyclohexane needs to be.
I cant see how the structure drawn is the enantiomer. The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. In the ring-flipping process C. Ask Question Asked 5 years 6 months ago. In a chair there are three carbons that are like mountain peaks red balls and three that are notches blue balls.
 Source: wikiwand.com
Source: wikiwand.com
Cyclohexane Conformation Wikiwand Viewed 2k times 2 begingroup I have two questions regarding this solution. The rest are considered as a twisted conformation. Most commonly encountered in the chair conformation of cyclohexane. The chair conformation of cyclohexane is not rigid. The CH2 bending frequencies. We can think of it as two chains mirror images one of the other containing atoms 1-2-3-4 and 1-6-5-4 with opposite dihedral angles.
 Source: researchgate.net
Source: researchgate.net
4 C 1 Chair Conformation Of A Hexopyranose And Newman Projections Of Download Scientific Diagram NB that your cyclohexane might not initially be in this conformation if you are very unlucky and have somehow managed to arrange the atoms in such a manner that they are closer to one of the other conformations than the chair one and in that case we will have to rearrange them to our desired conformation. Chair conformation chirality. NB that your cyclohexane might not initially be in this conformation if you are very unlucky and have somehow managed to arrange the atoms in such a manner that they are closer to one of the other conformations than the chair one and in that case we will have to rearrange them to our desired conformation. So that it looks exactly like the chair conformation shown on the left in Figure 4-33b. A high energy conformation of cyclohexane that occurs during ring flipping. The chair conformation is found to be more stable.
 Source: wikiwand.com
Source: wikiwand.com
Cyclohexane Conformation Wikiwand This specific conformation that we are going to look at in a moment is called the chair conformation. To build the chair conformation. In the boat conformation two of the substituents those on the bow and the stern if you will are brought close enough to each other to cause steric strain. The chair conformation in which the methyl group is equatorial is the most stable while the alternative chair conformation in which the methyl group is axial is 73 kJmol more energetic. If the tert-butyl group is placed in the axial bond then the chair has the highest energy or the least stable conformation. How can geometric isomerism arise due to.
 Source: slidetodoc.com
Source: slidetodoc.com
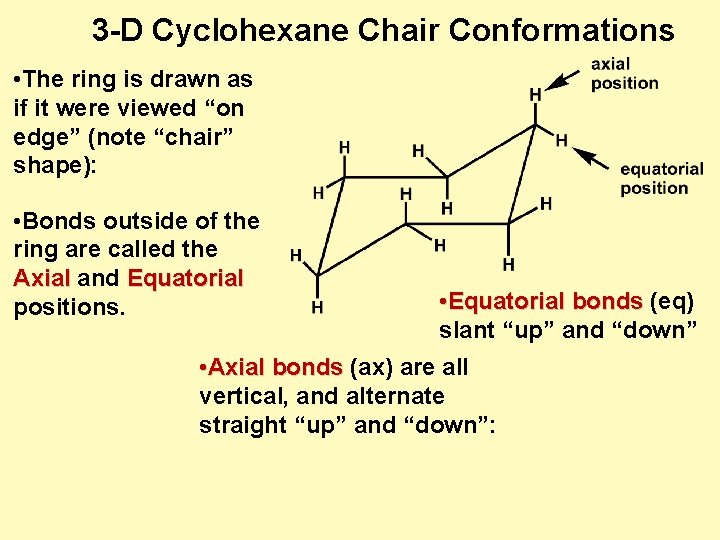
Ch 4 5 Cycloalkane Conformations I Cyclohexane Conformations In a chair there are three carbons that are like mountain peaks red balls and three that are notches blue balls. To build the chair conformation. Then you should familiarize yourself with the geometry of chair conformation. The bond angle in chair conformation is 1095 similar to the bond angle in a tetrahedral geometry. YOUR TURN Draw each line structure as a Haworth projection and each Haworth projection as a line structure including dashwedge notation. Set the Default Element to carbon and get into drawing mode.
 Source: researchgate.net
Source: researchgate.net
Chemical Structures Of Benzene Top And Cyclohexane Bottom Benzene Download Scientific Diagram An alternate conformation for a six-membered ring is called the boat. In fact cyclohexane take the chair conformation to achieve a bond angle similar to the tetrahedral geometry to eliminate angular strain. The CC stretching frequencies of the chair form are found to be higher than those of the planar ones. In the boat conformation two of the substituents those on the bow and the stern if you will are brought close enough to each other to cause steric strain. I cant see how the structure drawn is the enantiomer. The chair conformation of cyclohexane is not rigid.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
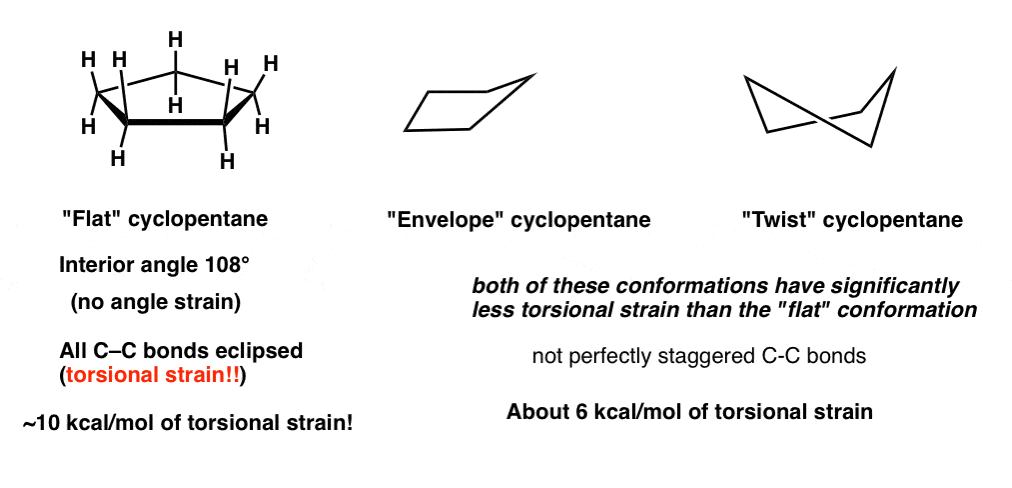
Cyclohexane Conformations Master Organic Chemistry Geometry vibration frequencies and IR absorption intensities of 6-radialene considering the planar and chair form. MedSchoolCoach expert Ken Tao will teach everything you need to know about chair conform. Chair is the most stable conformation of cyclohexane. Set the select level to Atoms. Although the hydrocarbon cyclohexane is typically drawn as if it were flat in reality the structure is not flat at all. Eclipsed bonds bonds gives torsional strain to.
 Source: pinterest.com
Source: pinterest.com
Organic Chemistry Educational Infographic Cyclohexane And Chair Conformation Video Organic Chemistry Chemistry Educational Infographic MedSchoolCoach expert Ken Tao will teach everything you need to know about chair conform. An unstable conformation of cyclohexane is the boat conformation which is 71 kcalmol higher in energy than the chair form. Chair conformation chirality. Active 5 years 6 months ago. So that it looks exactly like the chair conformation shown on the left in Figure 4-33b. Most of the time the structure exists in what is called the chair conformation.
 Source: organicchemistrysolutions.com
Source: organicchemistrysolutions.com
Stereoisomeric Conformations Different Perspectives Achiral Stereoisomers And Meso Compounds Organic Chemistry Solutions YOUR TURN Draw each line structure as a Haworth projection and each Haworth projection as a line structure including dashwedge notation. Although the hydrocarbon cyclohexane is typically drawn as if it were flat in reality the structure is not flat at all. We can think of it as two chains mirror images one of the other containing atoms 1-2-3-4 and 1-6-5-4 with opposite dihedral angles. Set the select level to Atoms. This geometry of chair cyclohexane conformations is generally preserved when the hydrogen atoms are replaced by halogen atoms such as fluorine chlorine bromine and iodine. YOUR TURN Draw each line structure as a Haworth projection and each Haworth projection as a line structure including dashwedge notation.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
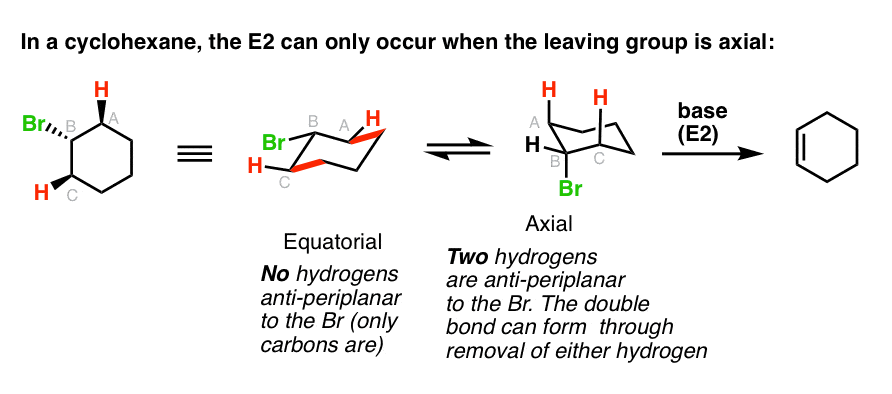
Antiperiplanar Relationships The E2 Reaction And Cyclohexane Rings Most of the time the structure exists in what is called the chair conformation. In a chair conformation the bond angles for each carbon are about 109 degrees so the tetrahedrals are pretty close. The chair conformation of cyclohexane is not rigid. The CC stretching frequencies of the chair form are found to be higher than those of the planar ones. The peaks have axial up and notches have axial down. This chair conformation is the lowest energy conformation for cyclohexane and other six-membered rings.

A The Chair Conformation Of Cyclohexane B Representation Of The Download Scientific Diagram It can convert to a twist boat comformation and then to a new chair conformation in a process termed ring-flipping as shown Figure 614 not all the hydrogens are shown for clarity. So that it looks exactly like the chair conformation shown on the left in Figure 4-33b. We can think of it as two chains mirror images one of the other containing atoms 1-2-3-4 and 1-6-5-4 with opposite dihedral angles. Building Chair Cyclohexane. If the tert-butyl group is placed in the axial bond then the chair has the highest energy or the least stable conformation. Chair is the most stable conformation of cyclohexane.
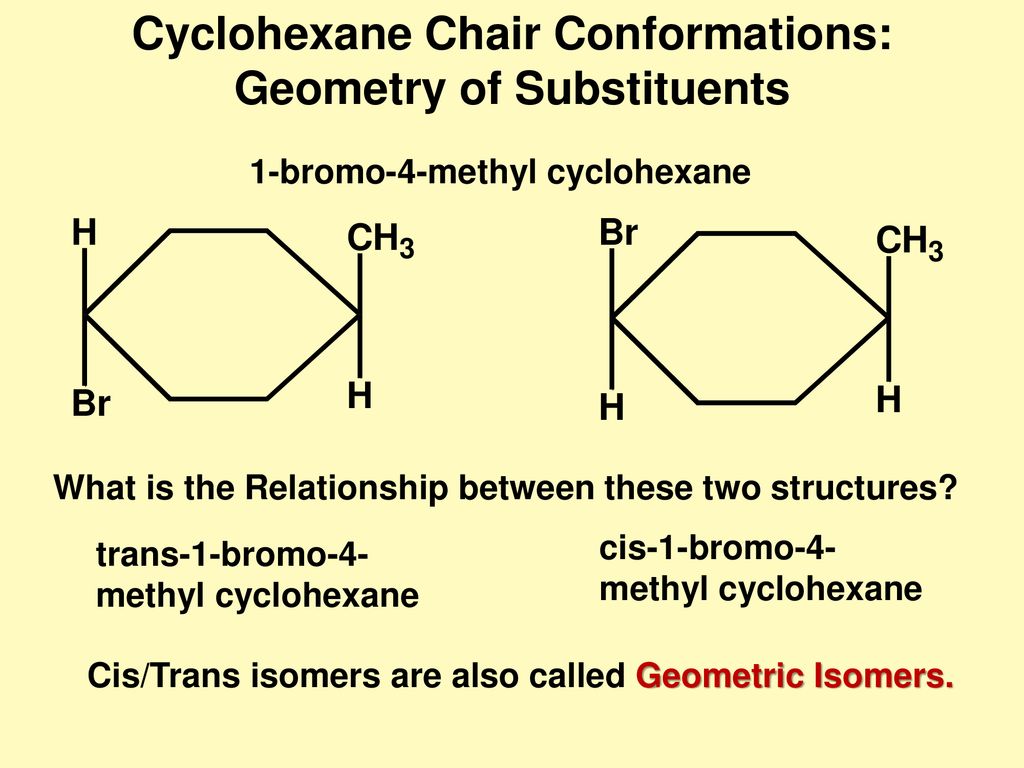 Source: slideplayer.com
Source: slideplayer.com
Ch 4 5 Cycloalkane Conformations I Ppt Download Ask Question Asked 5 years 6 months ago. 180 pm Boat conformation is less stable than the chair. It can convert to a twist boat comformation and then to a new chair conformation in a process termed ring-flipping as shown Figure 614 not all the hydrogens are shown for clarity. Most of the time the structure exists in what is called the chair conformation. Choose Labels on the Display menu and label the atoms by number. The distance from atom 1 to atom 4 depends on the absolute value of the dihedral angle.
 Source: researchgate.net
Source: researchgate.net
0 88 Hz And 0 01 Hz In The 4 Twisted Chair Conformation In Order Download Scientific Diagram Playing with a model can help in learning that. How can geometric isomerism arise due to. It can convert to a twist boat comformation and then to a new chair conformation in a process termed ring-flipping as shown Figure 614 not all the hydrogens are shown for clarity. 180 pm Boat conformation is less stable than the chair. In the ring-flipping process C. A high energy conformation of cyclohexane that occurs during ring flipping.
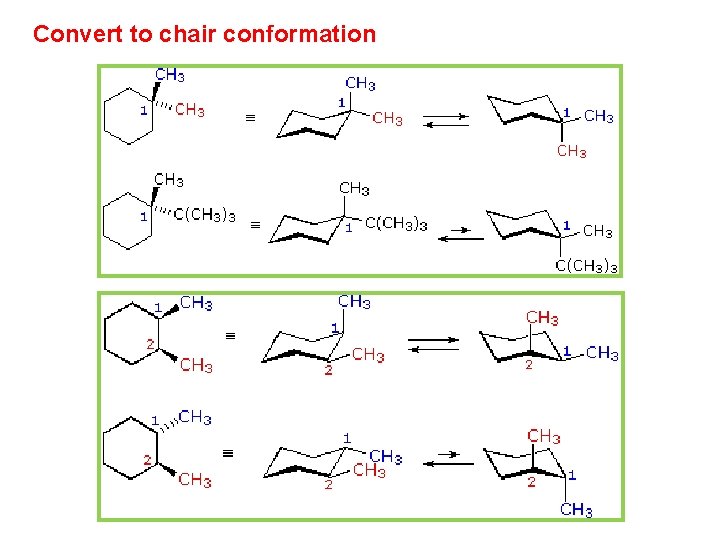 Source: slidetodoc.com
Source: slidetodoc.com
Chapter 4 Alkanes Alkenes And Alkynes Nomenclature Conformational The first form of cyclohexane you build is the chair form. An unstable conformation of cyclohexane is the boat conformation which is 71 kcalmol higher in energy than the chair form. Here are videos where I full explain the chair conformation and why it happens along with its stereoisomerism. The bond angle in chair conformation is 1095 similar to the bond angle in a tetrahedral geometry. To build the chair conformation. Chair is the most stable conformation of cyclohexane.
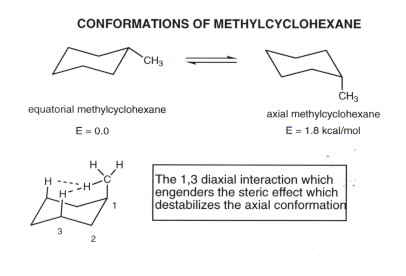 Source: research.cm.utexas.edu
Source: research.cm.utexas.edu
Cyclohexane Conformational Analysis This chair conformation is the lowest energy conformation for cyclohexane and other six-membered rings. Choose Labels on the Display menu and label the atoms by number. This specific conformation that we are going to look at in a moment is called the chair conformation. I cant see how the structure drawn is the enantiomer. Viewed 2k times 2 begingroup I have two questions regarding this solution. Set the select level to Atoms.
 Source: researchgate.net
Source: researchgate.net
Plausible Configurations Adopted By The L Pipecolic Acid Molecule Download Scientific Diagram Actually these bonds are a bit wobbly too enough for the carbons to wobble between boat and chair conformations on occasion. This chair conformation is the lowest energy conformation for cyclohexane and other six-membered rings. In fact cyclohexane take the chair conformation to achieve a bond angle similar to the tetrahedral geometry to eliminate angular strain. We can think of it as two chains mirror images one of the other containing atoms 1-2-3-4 and 1-6-5-4 with opposite dihedral angles. This geometry of chair cyclohexane conformations is generally preserved when the hydrogen atoms are replaced by halogen atoms such as fluorine chlorine bromine and iodine. Without flipping the chair simply rotate the molecule again until it looks like the chair conformation on the right.
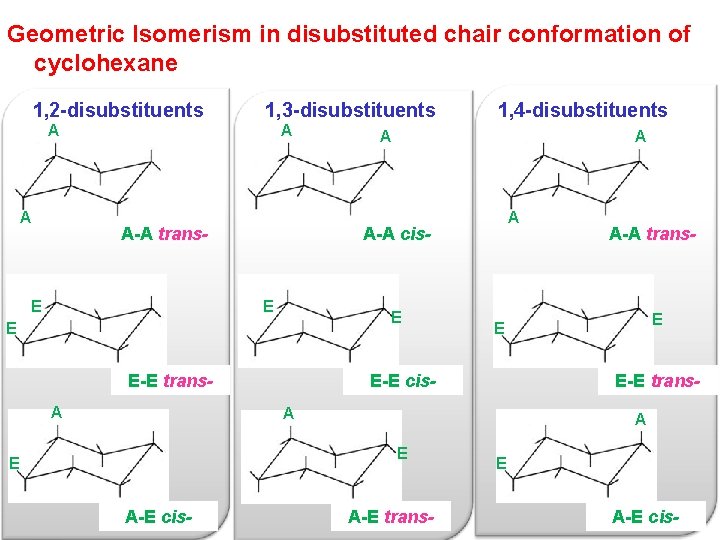 Source: slidetodoc.com
Source: slidetodoc.com
Chapter 4 Alkanes Alkenes And Alkynes Nomenclature Conformational However keep in mind that the geometry of the cyclohexane needs to be. YOUR TURN Draw each line structure as a Haworth projection and each Haworth projection as a line structure including dashwedge notation. The rest are considered as a twisted conformation. The chair conformation of cyclohexane is not rigid. In a chair conformation the bond angles for each carbon are about 109 degrees so the tetrahedrals are pretty close. What is the most stable chair conformation.

What Is Chair Conformation Quora An alternate conformation for a six-membered ring is called the boat. The Chair Conformation The stability data in Table 71 require that the bond angles in cyclohexane must be essentially the same as the bond angles in an alkanevery close to the ideal 1095 tetrahedral angle. The chair conformation of cyclohexane is not rigid. MedSchoolCoach expert Ken Tao will teach everything you need to know about chair conform. Without flipping the chair simply rotate the molecule again until it looks like the chair conformation on the right. The most stable conformation for cyclohexane.
 Source: researchgate.net
Source: researchgate.net
Conformation And Dihedral Angles Of Cyclohexene As Part Of The Tetralin Download Scientific Diagram The rest are considered as a twisted conformation. The chair conformation cannot deform without changing the bond angles or lengths. In fact cyclohexane take the chair conformation to achieve a bond angle similar to the tetrahedral geometry to eliminate angular strain. I cant see how the structure drawn is the enantiomer. Geometry vibration frequencies and IR absorption intensities of 6-radialene considering the planar and chair form. If all six torsions are near - 60 margin 30 it is considered a chair conformation.
Please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title chair conformation geometry by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.











